Methyl 2-Bromohexanoate: A Deep Dive into a Crucial Organic Intermediate
Historical Development of Methyl 2-Bromohexanoate
The journey of Methyl 2-Bromohexanoate stretches back to early synthetic organic chemistry, where halogenated esters played a fundamental role in modifying carbon skeletons for molecular design. Laboratories around the world churned out these molecules in efforts to create new pathways for drug discovery, specialty materials, and agrochemicals. Experimenters in the mid-1900s realized that by adding bromine atoms to acyl groups, they could generate reactive sites perfect for further transformation. This sparked regular use of compounds like Methyl 2-Bromohexanoate as reliable building blocks, especially since simple starting materials were widely available and the synthesis did not require exotic reagents.
Product Overview
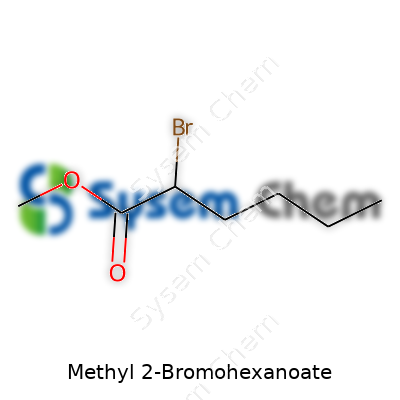
At its core, Methyl 2-Bromohexanoate is an alkyl ester featuring a six-carbon chain, carrying a bromine atom at the 2-position. This structural nuance gives it strong versatility in labs and industrial settings. It crops up as a white to pale yellow liquid, used in tailored syntheses across sectors ranging from medicine to new-age polymer science. Laboratories reach for it thanks to its manageable stability, straightforward preparation, and ability to serve as a precursor for more intricate compounds. Supply chains today deliver it in bulk for well-funded research groups, but small batches can still be synthesized in-house where custom modification is crucial.
Physical & Chemical Properties
Most chemists handling Methyl 2-Bromohexanoate notice a strong odor, an oily consistency, and reliable solubility in common organic solvents such as chloroform, dichloromethane, and ether. Its melting point sits low enough to ensure it remains liquid under standard ambient conditions. The molecular formula, C7H13BrO2, gives a molar mass just under 210 g/mol, which influences how it behaves in both analysis and reactions. The bromine atom grants reactivity often exploited in SN2 substitutions and various cross-coupling reactions. Its density eclipses water, making separation in multi-phase extractions simple. Chemical resistance leans toward acids and bases, but excessive heat or strong nucleophiles cleave the carbon–bromine bond, calling for measured reaction controls.
Technical Specifications & Labeling
Any container of Methyl 2-Bromohexanoate should carry identifiers like CAS Number 4334-19-6 and standardized labeling for hazardous organics. Standard purity hovers at 97% or greater in reputable chemical catalogs, with GC or NMR confirmation included in quality assurance documentation. Labels show hazard warnings for skin and eye irritation as well as for environmental toxicity. Transport requires firm-lidded amber bottles to minimize both light exposure and volatility. Batch numbers, lot tracking, and expiration dates all form part of prudent practice, not just for compliance but to pull data quickly if an incident arises or a batch goes off-spec.
Preparation Method
Preparation usually starts from hexanoic acid, which gets brominated at the alpha position via reagents such as bromine or N-bromosuccinimide under acidic or photochemical conditions. The intermediate 2-bromohexanoic acid reacts with methanol, often in the presence of a catalyst like sulfuric acid or HCl, to form the methyl ester. Extraction and fractional distillation purify the product. Yield optimization draws on careful temperature control, slow addition of bromine, and phase separation skills honed through lab experience. Mishandling—rushing the bromination, imprecise temperature—can result in side products or decomposition, which eat into recoverable yield and bring about impurities harder to purge downstream.
Chemical Reactions & Modifications
The bromine atom sits as an inviting target for nucleophilic substitution, so Methyl 2-Bromohexanoate becomes a springboard for custom molecules bearing amines, thiols, or alkoxides. It can seed cross-coupling reactions if the right catalysts are present. Researchers use it for chain elongation, ring closures, or introducing functionality onto larger frameworks. On a bench-top scale, I’ve found it plays well in Grignard reactions and Suzuki couplings, assuming scrupulous exclusion of moisture and careful monitoring of exotherms. In industry, chemo-selectivity and the ability to tailor reactivity drive the choice to use this ester over other halogenated substrates. Downstream applications can involve conversion into amino acids, secondary alcohols, or even complex lactones through relatively straightforward reaction cascades.
Synonyms & Product Names
Chemists searching for Methyl 2-Bromohexanoate might also come across trade names or synonyms such as 2-Bromohexanoic acid methyl ester, Hexanoic acid, 2-bromo-, methyl ester, or Methyl 2-bromo-n-hexanoate. Each name points to the same core chemical entity, but differences in regional naming conventions or chemical supplier branding sometimes cause confusion, especially for those new to international sourcing. Standardizing stockroom records helps avoid mixups, particularly within programs where dozens of ester derivatives turn up in weekly reaction planning.
Safety & Operational Standards
Hands-on handling calls for gloves, eye protection, and fume hoods thanks to strong odor and risk of inhalation or skin absorption. Spills and splashes should prompt cleanup with absorbent material and thorough soap-and-water scrubbing. Storage stays safest in cool, dry, segregated areas away from strong bases or moisture, which can break down the ester. Workers need safety training focused on potential for respiratory tract irritation or delayed allergic reactions. Regulatory paperwork—like SDSs—borrows from frameworks such as OSHA, REACH, and GHS, listing routes of exposure, symptoms, and emergency measures. In my experience, the most dangerous moments come not during careful use, but when protocols go ignored—or after hours, when less-experienced staff rush cleanup and push aside best practice.
Application Area
Methyl 2-Bromohexanoate pops up in organic synthesis routes aimed at pharmaceuticals, specialty polymers, and agrochemicals. Medicinal chemists value it for its ability to introduce tailored side chains or reactive centers with precision, often sparking developments in antifungal or antibacterial compounds. Its chain length gives materials scientists flexibility when crafting block copolymers with targeted flexibility or degradation profiles. In agricultural settings, it serves as an intermediate for herbicide and pesticide manufacture, offering a compromise between reactivity and environmental stability. The diversity of its transformations means it anchors both academic research and process-scale manufacturing alike, forging new pathways from bench to pilot plant.
Research & Development
Many innovation stories trace back to tweaks in the building blocks. Research teams keep trying new reaction approaches to reduce byproducts or boost atom efficiency in making derivatives of Methyl 2-Bromohexanoate. Catalysts tailored for greener, lower-temperature bromination might someday tighten safety margins and cut waste. In my own trials, switching solvent systems or varying catalyst loading opened unexpected doors, sometimes slashing purification work by days. Current projects lean toward discovering more sustainable methods for bromine introduction and shifting away from heavy metals in downstream chemistry. As regulatory pressure mounts, the chance to replace legacy techniques with leaner, cleaner options excites everyone invested in both performance and environmental protection.
Toxicity Research
Animal studies flag Methyl 2-Bromohexanoate for irritation potential, especially in eyes, respiratory tract, and on skin. Acute exposure studies point to reversible effects on contact, but repeated exposure may provoke dermatitis or sensitization in some people. Inhalation carries more risk than skin absorption according to most published findings, so good ventilation proves its worth every day in the lab. Environmental toxicity research spotlights risk to aquatic organisms if significant spills reach waterways. On-site treatment and spill containment get special attention in chemical plants to head off accidental release and its consequences for fish or invertebrates. Research pushes for improved hazard labeling and tailored PPE recommendations, especially with stricter oversight from national health agencies worldwide.
Future Prospects
Demand for precision intermediates like Methyl 2-Bromohexanoate rises as new therapies, smarter pesticides, and advanced polymers call for exact chemical building blocks. Green chemistry stays in the spotlight, driving interest in milder, safer preparation methods and renewable feedstocks. Digital tools like process modeling and AI-guided synthesis planning promise fresh insights into reaction optimization and risk reduction. The field sees increasing collaboration between academic groups chasing next-generation molecules and manufacturers refining cost, safety, and scalability under mounting regulatory expectations. The balance between chemical performance, workplace safety, and sustainability will shape the path of Methyl 2-Bromohexanoate and its cousins in global chemistry pipelines for years to come.
A Close Look at the Backbone
Methyl 2-Bromohexanoate’s name alone hints at its layout. Hexanoate forms the chain—think six carbons in a row, like links holding tight. Then comes “methyl,” which attaches as an ester group. Add in a bromine atom stuck to the second carbon, and that’s the core. Drawing from my days in undergraduate organic chemistry, seeing this arrangement helped untangle the jumble of letters and numbers. The base starts as hexanoic acid, then a methyl group swaps out hydrogen from the carboxylic acid, turning it into an ester. The bromine slips onto that second carbon (the alpha position), transforming the chain into something far more interesting chemically.
Chemical Structure Unpacked
Visualizing the molecule isn’t tough once you spot the pattern: CH3-CH(Br)-CH2-CH2-CH2-COOCH3. The methyl ester group sits on the tail, with bromine crowding the next-to-front position. Early in my research work, I learned that substituting atoms like bromine onto otherwise familiar chains ramps up both reactivity and function. Bromine, a hefty, electronegative presence, sets this molecule apart from its plain hexanoate relatives. Forget simplifications about “functional groups”—the difference is visible enough for most chemists with a felt-tip marker and a napkin.
Aside from structure, this compound catches the eye for its versatile roles in synthetic chemistry. The bromine acts a bit like a handle: it lets chemists swap that position for other atoms or groups with ease, using it as a springboard for building bigger, sometimes more valuable molecules. Not all esters give you that shortcut; this one does. Some classmates joked it acts like a Lego connector, always ready to snap on something new.
Why This Structure Matters in the Lab
The unique layout impacts how it behaves in reactions. Esters, including methyl 2-bromohexanoate, tend to show up where someone wants mild flavor or aroma, but putting a bromine on board moves it into a more reactive class. Chemists use it to create pharmaceuticals, agricultural compounds, and specialty materials. With that bromine on board, carbon at position two becomes a magnet for nucleophiles—molecules looking to swap places or build something more complex. In a synthetic sequence, picking out this compound can simplify steps, save time, and cut costs. I’ve seen countless projects stall for lack of a good leaving group—bromine here gives that edge.
Manufacturers benefit from its predictable reactivity, but safe handling draws attention because brominated compounds can pose risks. Regulatory guidelines keep labs on their toes about proper ventilation, storage, and disposal. I picked up careful habits after one spill in a teaching lab—the odor, the paperwork. Reading safety data sheets is time-consuming, yet stories like mine keep the point clear: these reagents deserve respect.
Looking Ahead: Responsible Chemistry
More researchers now ask what consequences follow routine methyl 2-bromohexanoate use. Environmental persistence and toxicity concerns push labs toward greener practices. Substitution with less hazardous reagents, developing recycling protocols, or designing safer reaction pathways arise as possible solutions. The field keeps shifting. Efforts to balance reactivity with responsibility steer synthetic chemistry toward safer, cleaner results, while still relying on classic structures. The molecule’s features—a defined backbone, reactive handle, real benefits—keep it in play, even as the industry seeks better methods and materials for tomorrow’s discoveries.
The Real Workhorse in Organic Synthesis
Methyl 2-bromohexanoate often comes up in conversations among chemists working with carbon chains and halogenated building blocks. For folks in organic labs, this one doesn’t show up by accident. Its chemical backbone rings a bell for anyone who has tried creating longer, more functional molecules without reinventing the wheel every time. The presence of both a bromine atom and an ester group serves as a jumping-off point for a staggering range of reactions.
Making Pharmaceuticals Start With Smart Building Blocks
Every pharmacist knows the journey from raw material to a shelf-ready drug can wind through dozens of steps. At several of these forks, chemists count on compounds that can transform without much struggle, keeping things manageable. Methyl 2-bromohexanoate shows up early on in the process of creating active pharmaceutical ingredients, especially those that call for a six-carbon backbone spiced up with a touch of halogen. This flexibility gives medicinal chemists a shortcut for turning out beta-branched acids, esters, and other motifs that tend to be hard to manufacture.
The ester’s reactivity lets researchers swap out groups, build rings, or lengthen chains. Tackling enzyme inhibitors or some fat-soluble drug candidates benefits from such a tool in the kit. It fits into steps that demand control, such as making prodrugs that unlock later in the body, or customizing a molecule’s solubility—both jobs that can make or break an experimental drug.
Bridging Fine Chemicals and Agrochemicals
The chemistry world doesn’t just turn out medicines. Agrochemical makers lean on building blocks just as much. Methyl 2-bromohexanoate handles this work by helping build complex herbicides, insecticides, and intermediates for plant protection. The brominated chain reacts well with nucleophiles, offering an entry to otherwise costly or time-consuming intermediates. Extension work in these supply chains opens new ground for more sustainable crop treatment, since efficiency on the molecular level translates to less waste and smarter use of raw materials.
Custom Synthesis and Research Applications
A lot of custom research projects live and die by how versatile their starting materials are. University teams and contract labs gravitate toward molecules like methyl 2-bromohexanoate for one reason—predictable chemistry with room to tweak. Swapping the bromine can link two building blocks, create new polymers, or set the stage for asymmetric synthesis, especially when aiming for enantioselective products with real-world applications.
In my own lab work, we used methyl 2-bromohexanoate to streamline syntheses that used to stumble over harsh conditions. With milder reagents, our reactions kicked off cleanly and avoided unraveling fragile groups added late in the game.
Real Hazards Demand Smart Handling
Anyone around halogenated esters understands the stakes. Compounds like this offer high yield and flexibility, but mishandling brings health risks—skin contact, inhalation, or spills can’t go unchecked. Proper storage, protective gear, and well-ventilated spaces let chemists harness the benefits without sacrificing safety. For those overseeing production or teaching, reinforcing these habits keeps everyone healthy and operations running.
Looking Forward: More Sustainable Practices
The tide has shifted toward green chemistry over the last decade. While methyl 2-bromohexanoate’s halogen is useful, labs look for ways to minimize hazardous byproducts and scale up with less impact. Changing reaction conditions, recycling solvents, and inventing cleaner transformation steps can take pressure off both budgets and the environment. This isn’t just wishful thinking—industry has real incentive to turn versatile pieces like methyl 2-bromohexanoate from merely useful to downright responsible.
Methyl 2-Bromohexanoate Quality: Why Chemists Pay Attention
Every organic chemist working with fine and specialty chemicals knows the headache that comes with variable reagent quality. For Methyl 2-Bromohexanoate, a compound used in pharmaceutical research, material science, and sometimes in fragrance synthesis, purity shifts can throw entire processes off. I remember working on a medicinal chemistry project that ground to a halt because what showed up in the bottle never matched the specs promised on the supplier’s website. Sometimes it came in looking pure by NMR, but a GC run told another story. Purity matters for yields, for safety, and for reproducible science.
Typical Purity Levels Found on the Market
If you check out five of the big-name chemical suppliers, you’ll see Methyl 2-Bromohexanoate most often advertised at a minimum 97% purity, with options for 98% or even 99% on occasion. Lower grades, like 95%, sometimes crop up, but they rarely satisfy research requirements. Direct experience taught me not to skimp on purity, especially in transition-metal catalysis or when isolating delicate intermediates. Impurities—brominated byproducts, trace water, or leftover acid—push reactions sideways, kill catalysts, or mess with product isolation. At the same time, absolute purity (above 99.5%) is rare for this compound unless you reach out for custom synthesis, which cranks up the price and lead time to something impractical for routine work.
Grade Definitions: Technical, Laboratory, and Research
Manufacturers typically sort the compound into technical, laboratory, or research grades. Technical grade sometimes contains more byproducts, traced back to large-scale production processes designed for bulk industrial applications, not bench chemistry. I deal nearly exclusively with laboratory and research grades. These hit a better purity—often beyond 97%. They usually come with GC or HPLC chromatograms and a certificate of analysis so you know what you’re getting. Still, things can slip. I’ve seen batches labeled “>98%” that didn’t hold up under LC-MS or when running sensitive reactions. This taught me that vendor reputation and QC transparency carry more weight than paper guarantees.
Why Purity Makes a Difference in Application
Pharmaceutical researchers rely on clean starting materials. Any contamination can mean failed or misinterpreted results, especially in multistep syntheses where errors cascade. For those synthesizing chiral molecules, even a small impurity in the alkyl bromide can create trouble downstream. On a personal note, a run with impure material created a headache once; crystallization failed and the product contained a persistent, nonvolatile impurity that defied easy removal. Sourcing higher-grade Methyl 2-Bromohexanoate solved the issue, though it cost double what my PI expected.
Stronger Sourcing Practices and Solutions
Companies serious about product consistency invest in proper purification: careful distillation, drying steps, solid QC with validated chromatography, and honest lot-to-lot transparency. Smart labs test in-house with NMR and GC to confirm composition before using any new bottle in a critical synthesis. For those in academia or biotech startups, it can help to build partnerships with reliable vendors and negotiate purity guarantees up front. There’s usually room to improve if the demand and volume justify it.
Takeaways for Practicing Chemists and Buyers
Experience working with Methyl 2-Bromohexanoate pointed me toward one main lesson: chasing the highest-purity grade pays off more than trying to save on reagent cost. Productivity, safety, and trust in the science all start with what comes out of the bottle. The marketplace will keep offering competitive grades, but only a few match both the price and purity needed for real research progress.
Why Storage and Handling Matter
People who work in labs know that careless storage can turn a pretty routine chemical into a real hazard. Methyl 2-Bromohexanoate packs power: a strong alkyl bromide and a hexanoic ester together in one bottle. It’s not something you want leaking or spilling around curious hands or pets at home, either. If safety slips, accidents happen, and suddenly a long day gets a lot worse. Whenever I've worked with it, I notice its unmistakable, irritating smell hanging stubbornly in the air, which isn’t just unpleasant; it's a red flag that this compound doesn’t belong in poorly ventilated corners.
Proper Storage—Ventilation and Isolation
Most organic chemicals don’t like light or warmth, and methyl 2-Bromohexanoate falls in line there. Forget any notion of tucking it away in a sun-drenched cabinet or beside an office heater. Keep it in a cool, well-ventilated, and dry spot. The best spot? A dedicated, lockable chemical cabinet, far away from food, drinks, and personal gear. If you’ve got flammable storage, check local regulations—some labs use explosion-proof fridges, mostly for peace of mind, though this compound doesn’t carry the same fire risk as classic solvents.
Over the years, I’ve watched coworkers leave chemicals with loose lids, sometimes with spills right on the label. Those little mistakes don’t get smaller over time. Tighten the cap immediately after pouring, and read the label before returning it to storage. Using secondary containment, like a plastic tray beneath the bottle, saves cleanup trouble if anything leaks.
Smart Handling—Personal Protection Every Time
Ask anyone who’s been splashed by an alkyl bromide, and they’ll talk about skin, eyes, and airways burning within seconds. Direct contact leaves skin red and sore, and inhaling its vapors gets you coughing by the fume hood. My standard uniform starts with a fitted lab coat, a good set of nitrile gloves—double layering can save you some worries if you’ll work for a while—and solid goggles. Always handle open bottles inside a chemical fume hood, never on a bare lab bench.
Gloves and goggles aren’t optional, and neither is a respirator if the hood isn’t drawing well. Spills can stick around in carpets and wood, so keep to hard, cleanable surfaces. Every so often, I see someone cut corners, working outside the hood because it’s “just a quick transfer.” Sooner or later, that risk catches up.
Disposal and Spill Response—Don't Wing It
Disposal doesn’t mean pouring leftovers into a drain. Facilities offer organic waste disposal services, and any waste, even a contaminated glove, should land in the designated container. Emergency spill kits belong within arm’s reach—these kits work fastest if folks learn how to use them before spill day. For a minor leak, neutralizing powders, absorbent pads, and good ventilation will do the trick. If you get a splash on yourself, rinse fast with water, tell a supervisor, then report for medical care.
Solutions—Make Training Real
Practical, real-time training helps much more than a stack of outdated MSDS printouts. Simulate a spill or glove tear at a lab meeting, let people practice without pressure, and they’ll move faster during the real thing. Posting quick-reference safety sheets by the entrance makes the right steps impossible to miss. Safety culture comes from routine: every time a new bottle gets opened, or an old one moved, repeat the basics, and keep everybody around a whole lot safer.
Understanding the Chemical
Methyl 2-Bromohexanoate pops up in labs as an intermediate in organic synthesis. With its sweet smell and clear liquid look, it might trick some people into taking it lightly. Here’s the truth: its chemical nature makes it potentially hazardous to our skin, eyes, and even organs if not treated with caution.
Health Hazards Worth Noting
Let’s start with contact. Skin exposure can lead to irritation or worse, depending on the duration and concentration. Eye splashes rarely end well—burning and redness kick in right away. If someone breathes in the vapors, coughing and throat irritation often follow. Swallowing a small amount isn’t a fate most want to experience either: nausea and abdominal pain show up fast. Longer or repeated exposures increase the risks. A friend who works in research once ignored gloves for a quick test—he walked away with days of painful dermatitis. It’s just not worth it.
The Fire and Environmental Angle
This ester can burn, with brominated organic compounds adding a layer of toxicity to the smoke. In a fire, breathing the fumes causes much bigger problems than just a sore throat; toxic gases may threaten first responders and bystanders alike. Spilling methyl 2-bromohexanoate near drains doesn’t just waste money—it risks local water habitats. Smaller aquatic life can’t always survive the contamination.
Project Safety Starts with Preparation
Young chemists sometimes rush into experiments, eager to move the project forward. In some labs, safety data sheets gather dust on a shelf. Reading these before opening the bottle means fewer surprises. Proper fume hoods can trap stray vapors. Good, tight-fitting gloves—nitrile or neoprene—protect skin and should be standard. Safety goggles with splash protections matter more than most people realize until they see an accident happen.
Storing and Disposing the Right Way
Team members keep this chemical away from heat or sunlight. Simple shelving isn’t enough. Corrosive-proof cabinets reduce the risk of unplanned reactions. I once visited a facility where a minor leak in a poorly sealed container turned the cabinet into a sticky hazard zone. Having the right containers cuts down cleanup and danger. Used rags, gloves, and empty containers belong in secure waste bins. Dumping left-over solutions down the drain is almost always illegal—licensed chemical waste contractors should handle disposal.
Planning for Mistakes
Accidents still happen even with planning. Eye-wash stations and safety showers right inside the work area make a real difference when things go wrong. Quick action—flushing eyes or rinsing off skin—shaves off long-term damage. Every worker should know where to find these on day one. Emergency phone numbers posted in the lab save minutes that could mean everything in a crisis.
The Role of Training and Culture
No matter how modern the facility, safety comes from a culture of honest discussion and routine review. I remember my supervisor in grad school drilling procedures into us every month. Boring as it felt, accidents never became headlines, and everyone left with their health. Training sessions aren’t box-ticking exercises—they make sure people respect the risks and keep each other accountable.
Looking Ahead: A Smarter, Safer Lab
Smarter procedures and real respect for the hazards turn a risky compound into a regular tool. Science and industry have taught that shortcuts never pay off. Chemicals like methyl 2-bromohexanoate demand good habits, vigilance, and teamwork. People make the difference—the right knowledge and preparation turn potential disasters into another routine day at work.