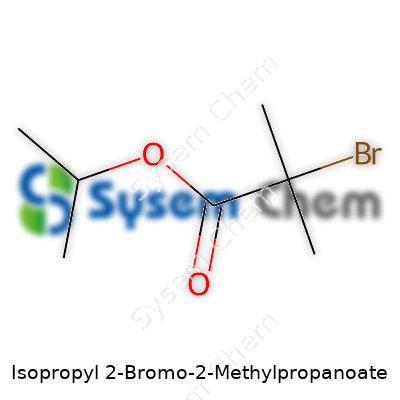
Isopropyl 2-Bromo-2-Methylpropanoate: A Down-to-Earth Look at a Key Chemical Building Block
Historical Development
Chemists began exploring halogenated esters seriously as far back as the 1960s, searching for stable but reactive intermediates—molecules that let you add or swap groups efficiently. Isopropyl 2-bromo-2-methylpropanoate came about in the race to develop better atom transfer radical polymerization (ATRP) initiators and serve the growing demands of specialty synthesis. The field kept shifting, but this compound stood out for its balance: the flexible isopropyl group paired with a reactive alpha-bromo center. In old lab notebooks, you’ll see this structure popping up once organic chemists realized that bromoesters, compared to chloroesters, offered easier engagement with copper and iron catalysts, which took off in the 1990s. That’s how one sees so many polymers and specialty molecules relying on this bromoester scaffold.
Product Overview
Isopropyl 2-bromo-2-methylpropanoate brings together elements of volatility and handling safety, with decent shelf stability. Suppliers lean toward the colorless liquid grade, typically sold in amber glass or fluorinated HDPE to keep light or stray water from spoiling the batch. If you ever meet a bottle, the telltale sharp ester odor will leave an impression. Beyond run-of-the-mill lab work, this ingredient has become essential for research groups and small polymer startups, who often keep a half-used bottle close at hand alongside their other bromoesters.
Physical & Chemical Properties
This ester weighs in with a molecular formula of C7H13BrO2, boiling at roughly 170-172°C at standard pressure and melting below room temperature. The density clocks in around 1.28 g/cm³, which feels noticeably heavier than water if you’ve handled it in the lab. Look up its refractive index, and you’ll see values pushing 1.437–1.444—helpful if you work with analytical chemists. Isopropyl 2-bromo-2-methylpropanoate dissolves into most organic solvents: ether, acetone, and even some aromatic hydrocarbons. It stays away from water if you want it to last. Its main party trick sits at the bromine—they call it a “labile” spot, where the bromine can hop off in controlled circumstances, a vital move for chain initiation and modifications.
Technical Specifications & Labeling
Most bottles sport a purity spec of 98% or better, checked by gas chromatography. Labels should warn of both the flammability and the risk of inhalation or skin contact, with hazard codes matching GHS standards. The packaging stays small—100mL to 1L volumes are common—because this isn’t a bulk commodity. Safety data sheets spell out UN number 3278, pointing to ester-flavored dangers with a whiff of halogen risk.
Preparation Method
Lab chemists favor a classic SN2 approach: grab 2-bromo-2-methylpropanoic acid, then treat it with isopropyl alcohol in the presence of a strong acid (sulfuric or p-toluenesulfonic). That protonates the acid, sets up the esterification, and gets the job done with minimal byproduct. Large-scale manufacturers stick with similar strategies, but they’ll dial up purity checks, add continuous extraction washes, and chase out traces of acids and leftover alcohols. The method’s appeal comes back to the starting material’s ready availability and the smooth reaction path—little need for exotic reagents or conditions.
Chemical Reactions & Modifications
The chemical backbone lets organic chemists unleash a range of transformations. The bromo group at the alpha position replaces smoothly with other nucleophiles, such as thiols, amines, or even stabilized carbanions, making it a flexible intermediate for custom monomer design. In ATRP, it serves as the leaving group that starts controlled polymerizations, while also playing into Giese reactions for new C–C bonds. Some teams fashion derivatives by swapping out the isopropyl ester for bulkier or more hydrophilic groups, customizing the initiator for various environments or chain-transfer chemistries.
Synonyms & Product Names
Running a search back through catalogs and literature, you’ll see this molecule passing under names like 2-bromo-2-methylpropionic acid isopropyl ester, isopropyl α-bromo-α-methylpropionate, or simply the moniker used by the likes of Sigma-Aldrich: IBMP Ester. Some refer to it by its registry numbers or as a ‘bromoester’, especially among old-timers. Each supplier might put their own branding twist—look for abbreviations like iPr-BMP or just iBrMP.
Safety & Operational Standards
Every person who’s spilled a drop knows to respect the hazards. This compound attacks skin and stings eyes; glove and goggle use isn’t optional. Vapors can irritate nasal passages if you huff too closely. Fire risk rides along given the flammable organic backbone, so most labs tuck it in a ventilated chemical cabinet, well away from heat. Disposal rules follow halogenated waste protocols. Training protocols hammer home the need for double containment during transfers and prompt cleanup if the stuff escapes its bottle.
Application Area
Polymer labs and research groups dive into this bromoester mostly as an ATRP initiator, especially for making designer materials with controlled molecular architectures. Paints and coatings industries take formulations down to the molecule by tailoring the initiator. A handful of pharmaceutical teams look to this as a handle to build up complex, branched APIs. Some surface scientists soak up its uses in grafting or surface-initiated polymerizations, locking a functional layer onto glass or metal. You also hear about its role in fine chemical contract research—one essential stop for chemists building out libraries of compounds for drug discovery or advanced materials.
Research & Development
Current research digs into new copper or iron complexes for ever-tighter ATRP control, while others push for greener, non-halogen routes—aiming to keep the chemistry agile but less hazardous. Patent filings show tweaks to the ester group, fulfilling new solubility or kinetic demands for high-value polymers. Academics lean into mechanistic studies, sketching out radical stability and chain transfer parameter details, all to sharpen the predictions for polymer end-group fidelity or dispersity in finished products.
Toxicity Research
Long-term studies on similar bromoesters bring some cautions. Acute studies in rodents show respiratory and dermal irritancy, while chronic data on similar chemicals suggest possible liver enzyme elevation with prolonged exposure. No cancer links in humans, but lab teams keep exposures low, erring on the side of caution given the reactivity profile. Regulatory circles, including REACH in the EU and TSCA in the US, urge manufacturers to submit periodic data, especially on downstream degradation products, to avoid unanticipated health questions down the line.
Future Prospects
With specialty polymers in high demand—from flexible electronics to stealth coatings—the need for precise initiators won’t fade. Green chemistry shifts could drive innovations that swap out the bromine for less persistent alternatives, or that knit bio-based esters into the process. Sequencers and automation now map out possible modifications in hours, not weeks, helping researchers build libraries around the core scaffold. As regulatory rules tighten, big chemical firms and startups alike will invest in safer handling, greener processes, and better personal protective tech, all to keep this versatile intermediate in the toolkits of future chemists.
Understanding Its Purpose in Laboratories
Isopropyl 2-bromo-2-methylpropanoate isn’t a household name, but it carries real importance in labs that focus on building and altering molecules. Researchers call it an “initiator”—a compound that sparks a chemical reaction, particularly in the production of special polymers. Polymers shape life as we know it, forming textiles, medical devices, packaging, and even the smartphones we scroll all day. This bromoester serves a critical job: it helps scientists kick off controlled radical polymerization, especially a process called atom transfer radical polymerization (ATRP).
ATRP gives chemists the precision to build custom plastics, coatings, or gels. Before ATRP took off, creating these materials often felt like a guessing game. Now, smarter initiators like isopropyl 2-bromo-2-methylpropanoate deliver control and predictability over the final product. It works in tandem with metal catalysts—copper is common—to guide how long the polymer chains grow and what features they pick up along the way.
Building Blocks For Advanced Materials
Polymers built through ATRP processes end up in far more than just plastic bottles. Companies develop custom hydrogels for medicine, responsive membranes for water purification, and specialty adhesives from the predictable chains spun from these reactions. Getting the initiation step right isn’t a minor detail—it decides the length, performance, and specialty traits of the end material. The isopropyl group hanging on this molecule helps set those early parameters that echo throughout the whole process.
Research teams exploring drug delivery depend on initiators like this, since the resulting materials must release drugs at the right moment or remain intact until reaching their target. Without these reliable starting materials, entire industries risk producing waste, using more energy, or struggling with inconsistent outcomes, which can hurt costs and even safety.
Responsible Handling Matters
With every upside comes new responsibilities. This compound, like most halogenated organics, can cause harm if handled carelessly. The bromo group makes it reactive—great in a controlled flask, not so great if spilled or inhaled. Proper storage, use of personal protective equipment, and waste disposal protocols become non-negotiable. Skipping safety shortcuts can expose researchers to health hazards and the wider environment to contamination. Each bottle needs respect, just like any chemical that packs potent reactivity.
Improving Access and Practices
For academic labs or companies working outside giant chemical hubs, sourcing high-purity initiators sometimes creates headaches and higher costs. Sharing resources, partnering with reputable suppliers, and investing in local production are fixes worth pursuing. There’s added value in institutions offering training for safe handling, not just in paperwork, but through hands-on demonstrations. Regulators can support research by keeping guidance clear and updated, making sure all stakeholders remain informed on both best practices and the latest science.
Looking Ahead
Chemistry sits at the crossroads of discovery and responsibility. Isopropyl 2-bromo-2-methylpropanoate, though its name is a mouthful, plays an outsized role in precision plastics and pharmaceuticals. Keeping an eye on safety, supply, and smarter training will help researchers harness the full promise of this molecule—while avoiding problems that can set progress back or risk well-being.
No One Likes an Explosive Mess
Chemicals like Isopropyl 2-Bromo-2-Methylpropanoate can spark more headaches than solutions if you don’t handle storage right. I’ve seen labs make simple mistakes that turn costly or, worse, dangerous. Lab safety manuals, paperwork from suppliers, and the Chemical Safety Board all point to the same thing: temperature and environment keep things stable or send things sideways.
Temperature: Cool, Not Cold
From working years in a modest research lab, shelving this chemical with the regular stuff always raised eyebrows. Heat speeds up problems—decomposition or unwelcome reactions. Best to stay under 25°C (77°F). No, you don't need a deep freeze, just a dry, cool cupboard away from sunlight and heavy sources of heat.
Humidity: Keep It Low
Watch out for moisture like you watch out for spills. I once saw a bottle sweating in a damp corner shelf—thankfully noticed before anything warped or leaked. Damp settings can nudge chemicals toward breakdown or even corrosion of their own containers. Always aim for a dry, ventilated room, and skip window ledges or ground-level storage where flooding or rain leaks could surprise you.
Light: No Sunbathing Required
Sunlight messes with more than just your skin. Chemicals in clear or even shaded bottles break down, discolor, or react in ways you probably won’t see coming. A dark or opaque storage cabinet gets the job done.
Sealing and Labeling: Details That Matter
I’ll admit, rushing to close bottles with a flimsy screw-on cap and scribbled label happened more than once. But leaks, fumes, and mix-ups all show up fast. Use tight, chemical-resistant lids. Clear, printed labels beat handwriting when things get hectic.
Avoiding Reactions: No Neighborly Sharing
Putting reactive chemicals near something incompatible is never worth the risk. Acids, strong bases, oxidizers—they all deserve their own shelves. I learned early on to always check for chemical storage charts posted in the lab. Potential reactions bring fires or toxic gases. If in doubt, keep Isopropyl 2-Bromo-2-Methylpropanoate by itself, rather than tempting fate.
Emergency Plans: Not Just a Form
You never know who might spill or drop a bottle. Spill kits, clean air, and easy-to-follow instructions make a huge difference. The time a new intern knocked a container, quick thinking and easy access to proper gear turned a mess into a teaching moment instead of a disaster.
Supplier Advice and Regular Checks
Suppliers usually have storage guidelines ready—sometimes in small print, sometimes prominent for good reason. Always look these over, even if you think you know the routine. Set reminders for stock inspections. Leaks and expired chemicals accumulate if no one checks regularly.
Trust Experience
Years of handling flammable or sensitive materials taught me there’s no shortcut with safe storage. Conditions protect not only those handling the chemical but also the research investment itself. Whether you’re managing industrial shelves or a single bottle in a teaching lab, sticking to the essentials saves lives, time, and resources.
Understanding the Risks in Simple Terms
Walking into a chemistry lab, there’s always a faint smell of something unfamiliar in the air. Some of those odors come from chemicals with real bite if you treat them casually. Isopropyl 2-Bromo-2-Methylpropanoate stands in that group. You look at the bottle, see complex phrases on the label, and maybe a bold red diamond. This compound gets regular use in synthesis, especially when folks work on producing specialty molecules or pharmaceuticals. Most people outside the chemical sector don’t think about it, but the story really matters for those of us handling or storing chemicals for a project.
Short-Term Effects Aren’t Little Things
If this compound hits your skin or eyes, it doesn’t quietly slip away. Expect redness and irritation, maybe more. Accidentally breathing in fumes—never pleasant—can leave you coughing, dizzy, and wishing for fresh air. Spilling some on your hands could lead to stinging right away. In a poorly ventilated room, even the person nearby might catch a whiff and start feeling woozy. The data lines up with what many have found in the real world: direct contact feels uncomfortable, repeated exposures only amplify the issue.
Long-Term Exposure? Risk Grows
Nobody lines up for chronic chemical exposure. Over time, repeated contact with chemicals like Isopropyl 2-Bromo-2-Methylpropanoate raises a person’s chance of skin problems, breathing trouble, and even more serious outcomes. Some bromo-compounds can break down into compounds that do more damage, messing with airway function or causing organ stress if too much gets absorbed. Lab studies show animals exposed regularly to similar compounds start stacking up internal damage—think liver and kidney trouble or worse. We don’t always see people facing the same effects right away, but science says being careful and keeping exposure minimal protects health in the long run.
Solid Science Supports the Precautions
Regulators treat chemicals with bromo groups seriously, and not without reason. Workplace guidelines frequently call for gloves, tight-sealing masks, and well-ventilated hoods when handling this compound. The National Institute for Occupational Safety and Health (NIOSH) keeps lists of chemicals that require caution, spotlighting compounds like this one. The Material Safety Data Sheets (MSDS) for Isopropyl 2-Bromo-2-Methylpropanoate lay out eye, skin, and breathing risks in plain text. Health agencies urge extra vigilance not just in laboratories, but in every spot this chemical shows up.
Keeping Ourselves and Others Safe
No fancy gear makes up for smart habits. Washing up after handling, labeling containers with clear warnings, and never skipping eye protection—those habits matter each time. Facilities see fewer accidents where people don’t cut corners or take shortcuts. Sharing stories with coworkers, reminding each other to wear proper gear, doubles as early-warning and backup for everyone. I once saw a student try to clean up a spilled sample with bare hands—he spent the afternoon getting checked out at campus health, then came back much wiser.
Solutions Are Clear and Effective
Training brings risk down quickly. Investing in safer containers, fitting labs with extraction fans, and making good SOPs the norm—all belong on a supervisor’s checklist. Keeping up with the latest research, swapping out hazardous reagents when green chemistry options become available, nudges workplaces closer to real safety. I walk into a lab with respect and a checklist; a tidy bench and clear labeling do as much to keep people safe as a robust fume hood.
Why Care About This Chemical?
Anyone who spends time in a lab or on a production line knows that cutting corners with chemicals often leads to trouble. Take Isopropyl 2-Bromo-2-Methylpropanoate — the name doesn’t roll off the tongue, but its risks straighten you up fast. This reagent pops up in organic syntheses, especially where precise control matters. It resembles other alkyl halides, notorious for their potential health hazards.
Understanding the Risks
A lot of compounds like this give off strong odors—the sort that make you want to step back. Vapors from Isopropyl 2-Bromo-2-Methylpropanoate don’t just smell strong, they can irritate your nose, throat, and lungs. Touching it without gloves can mean red, itchy skin, maybe even a rash. Eyes exposed to splashes often end up red and watering. Swallowing or breathing in too much may cause headaches, dizziness, and nausea. Chronic exposure sometimes brings long-term health issues, including lasting skin sensitivity or worsening asthma in people who already live with respiratory conditions.
My Take on Lab Precautions
Working in academic research, I’ve seen too many folks rush through chemical handling. The best professionals slow down and do things right. Whenever I work with this compound, I grab nitrile gloves, a chemical splash apron, and goggles that seal around my eyes. Standard disposable gloves sometimes fail after a short exposure, and latex doesn’t hold up long. Nitrile gloves last longer with halogenated reagents. I double-check my fume hood, making sure it pulls air well and nothing blocks the airflow. Keeping a spill kit nearby isn’t just a rule in our lab – it’s a real need. I remember one mishap, a minor spill, where quick use of absorbent pads helped avoid a major headache.
Handling and Storage Tips
Pouring straight from the bottle means trouble; the risk of splashes grows with every unsteady hand. I always use dispensing pipettes or an appropriate pump. I store the bottle in a tightly sealed container, labeled in plain language along with the hazard pictogram. Stashing it below eye level reduces the risk of dropping the bottle or a fume rush on opening. Our chemical fridge is dedicated only to volatile and reactive materials; I never mix incompatible chemicals on the same shelf. With Isopropyl 2-Bromo-2-Methylpropanoate, keeping it far from heat sources, acids, or bases prevents unwanted reactions or pressure buildup.
Responding to Spills and Exposure
Minor spills don’t stay minor if ignored. I always cover the spill with absorbent material while suited up, clean the area with water, and ventilate if odors linger. Exposed skin gets washed right away under running water—no shortcuts. If it gets in the eyes, fifteen minutes of flushing at an eye wash station is non-negotiable. Emergency numbers sit right by our workstation, not buried in a binder.
Building a Culture of Safety
Trust grows in a group when everyone watches out for each other. Sharing real stories about near-misses makes the need for careful handling more real than signs on the wall. Regular training, not just a yearly quiz, ensures new team members know safety isn’t optional. Keeping open lines of communication about accidents or worries means nobody has to handle risks alone.
Final Thoughts
A strong safety culture saves trouble, keeps people healthy, and protects research. Respect for chemicals like Isopropyl 2-Bromo-2-Methylpropanoate comes from habit, not fear. Once you commit to the right precautions—solid PPE, working fume hoods, quick cleanup, good labeling, and strong teamwork—the risks start shrinking, and the focus goes back where it belongs: doing solid, reliable work.
Trust Matters: Why Purity Makes or Breaks a Shipment
Working with chemicals such as Isopropyl 2-Bromo-2-Methylpropanoate means details are everything. In research, just a small impurity in a reagent can kill weeks of effort. In industry, any unknown contaminant can throw a wrench into a production run. I’ve spent enough time on both sides of the lab bench to see how a batch of inconsistent material causes expensive headaches, missed deadlines, and plenty of stress for folks further down the line.
Companies that supply this ester tend to list purities ranging from around 97% to over 99%. For many non-critical uses, 97% works. In pharma research, nobody takes chances on that last two percent. In my own experience, discussions around purity grades usually turn into debates about cost, but that last percentage point sometimes means the difference between success and hours poured down the drain.
What the Specs Mean—And Why They Matter
The grade shows what’s in the bottle. It’s not just about the percentage of the main chemical. People ask about water content, color, acidity, and specific gravity. Residues from bromination or isobutyric acid can mess with reactions. If specs are tight—say, water under 0.5%, colorless or pale yellow, and minimal side products—I tend to breathe easier. A good certificate of analysis gives me confidence I’m not gambling with my results.
Using something with 99% purity and proper handling means my reaction behaves the way textbooks predict. Fewer odd results, fewer cleanup steps, and more time spent pushing the science forward rather than babysitting columns or troubleshooting. Even later on, say in peptide synthesis or for controlled radical polymerizations, everything relies on reproducibility, and that starts with solid, well-defined starting material.
Sources and Red Flags
Plenty of labs lean on big suppliers because those companies are more transparent. I keep track of certificates and batch numbers. Sourcing from lesser-known suppliers brings risks. Batches differ, documentation gets spotty, and that leaves me guessing about what’s actually going into my flask. I’ve seen people try to cut corners by going for bargain options and it often bites them. A so-called 98% bottle hiding a few percent of leftover solvent or byproduct means purity claims fall apart in practice.
What to Watch Before Signing a Purchase Order
Purity isn’t just a number, it’s trust. Ask for recent HPLC or NMR data, not just an invoice stapled to a bottle. If a supplier hesitates to share a data sheet, that’s a warning sign. Even better, check that their product specs match the needs of your particular application. For anything heading into animal studies or pilot manufacturing runs, firms pay up for validated, traceable supply chains.
Safety plays a role, too. Lower-purity material can bring surprises: strange odors, unexpected reactions, or difficult waste disposal. Folks using this ester in complex setups, maybe for ATRP or as an acylating agent, know that the extra dollars for certified high-purity can save much more in labor and materials later.
Better Practice, Fewer Headaches
Setting up a clear, straightforward vetting process for suppliers saves time and money. I ask for batch-specific data and double-check against published values. In my lab, we avoid rerunning experiments by sticking with brands and lots that prove reliable. Maybe the big players don’t offer the cheapest bids, but buying peace of mind counts, especially if failure is not an option.