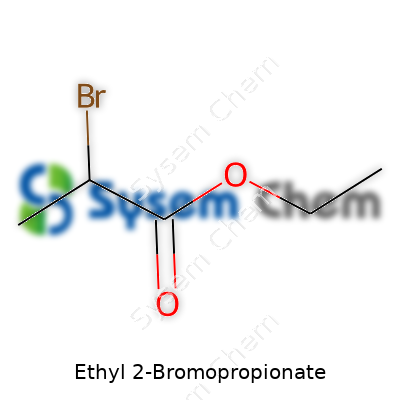
Ethyl 2-Bromopropionate: A Close Look at a Crucial Chemical Intermediate
Historical Development
Ethyl 2-bromopropionate entered the lab and industry toolkit decades ago, riding the wave of organic synthesis innovations from the early 20th century. Chemists looking for efficient ways to build carbon frameworks stumbled upon its strengths. As the chemical industry moved from coal tar and sugar-based feedstocks toward more advanced halogenated molecules, researchers started using ethyl 2-bromopropionate as a robust carbon–carbon building block. Its clear structure and reactivity made it useful beyond academic research, reaching out into commercial pharmaceutical and agrochemical production. By the 1960s, it had found steady use in making tailor-fit molecules for new agricultural chemicals and medical synthesis routes.
Product Overview
Recognized as an organobromine compound, ethyl 2-bromopropionate’s appeal comes from its simplicity and versatility. It has a modest carbon skeleton, an ester group for reactivity, and a bromine atom that opens doors for substitutions. My first run-in with this compound happened during a lab-scale reaction that called for a specific α-bromo ester. It comes bottled as a colorless liquid. You’ll spot it in catalogues with the formula C5H9BrO2, and the smell isn’t overwhelming but certainly not something I’d linger over. Companies supply it with purity ranging from technical grade up through analytical specifications, packaged to prevent moisture uptake and contact with incompatible reagents.
Physical & Chemical Properties
Ethyl 2-bromopropionate stands out with a boiling point just below 150°C and decent solubility in common organic solvents. It skips over water, keeping moisture sensitivity modest, but enough to warrant care in storage. With a density north of 1.4 g/cm³, it sinks below water. The ester group keeps the molecule relatively stable under ambient conditions, but the bromine atom brings a punch, as you can see during nucleophilic substitution or elimination reactions. Its refractive index helps quality control labs check purity in real-time. The vapor pressure, though not excessive, calls for hood use and tight cap closures.
Technical Specifications & Labeling
You’ll see labels with the CAS number (535-11-5), batch details, hazard pictograms, and purity information. Top-tier suppliers note any stabilizers or residual acid content, as trace impurities can matter for scale-up. A typical assay goes above 98%. Labels also stress the flammability risk, the need for chemical goggles, and secondary containment. Regulatory identifiers, including UN shipping codes and hazard classes, show how chemicals move across borders or between labs. Country-specific labels go deeper on GHS warnings and emergency-handling steps.
Preparation Method
In the lab, production often draws on the Hell–Volhard–Zelinsky reaction: start with ethyl propionate (an ester), add bromine and a catalytic bit of phosphorus. Heat brings bromination to the alpha position—the cornerstone for the right substitution. Some commercial producers take it up a notch with continuous flow reactors, reducing hot-spot risks and unwanted byproducts. Classic plans call for simple extraction and vacuum distillation to isolate product, but on industrial scale, columns operate under reduced pressure to keep thermal decomposition at bay. I learned quickly that bromine control helps maintain yield and limits waste.
Chemical Reactions & Modifications
Ethyl 2-bromopropionate breaks away from being just an endpoint and instead acts as a springboard for wider synthetic playbooks. Nucleophiles take out the bromine, leaving behind a new C–X or C–C bond, making this molecule a reliable choice in enolate chemistry or the creation of chiral centers for pharmaceutical intermediates. Specialized uses bring Grignard reagents into the mix, or see it step up in Michael additions for more advanced molecular construction. The molecule’s reputation in the lab comes from repeatable yields and the ease with which bromine can be coaxed out and replaced. Dehydrohalogenation routes give it a path into alkenes, which are valuable in further transformations.
Synonyms & Product Names
Besides the main name, chemists run into a few alternate tags: ethyl α-bromopropionate, propanoic acid 2-bromo-, ethyl ester, or 2-bromopropionic acid ethyl ester. Global catalogs track these names for shipping and regulatory compliance, and anyone ordering from multiple countries ought to keep an eye on local nomenclature differences. In some sectors, project teams work with product codes sprinkled across documentation, making clear communication important at every turn.
Safety & Operational Standards
Handling ethyl 2-bromopropionate takes respect for its irritant properties and awareness that brominated organics can react unpredictably with nucleophiles. My early days brought training under the fume hood, diligent glove use, and surface decontamination. Labs and plants rely on fresh air exchange and spill prevention: floors stay clean, absorbents stay close. Breathing vapor harms the lungs, and skin contact risks dermatitis. Shipping rules match those for other volatile organics—chemical-resistant containers, clear labeling, emergency response plans, and in some facilities, continuous air monitoring. Waste regulations demand halogenated organic solvents never mix with standard hydrocarbon waste streams.
Application Area
Use cases spread across pharmaceutical development, agrochemical synthesis, and materials science. In my work, pharma groups use ethyl 2-bromopropionate to build up skeletons for active pharmaceutical ingredients, especially in steps where chiral intermediates matter. Specialty chemical companies apply this molecule for synthesizing flavors, fragrances, and polymer building blocks, as the manageable bromine makes quick work of introducing additional complexity. In agriculture, researchers target new herbicide scaffolds with the alpha-bromo ester functionality. The versatility opens doors for academic studies of reaction pathways, often leading to new synthetic discoveries.
Research & Development
Research teams push the limits on making reactions safer, faster, and more selective. Some groups trace safer bromination agents, trading elemental bromine for less aggressive sources. Green chemistry labs optimize solvents, moving toward low-impact alternatives and closed-loop cleaning cycles. New catalysts show promise for boosting yields and suppressing troublesome side reactions. My peers who monitor R&D note a steady rise in publications exploring asymmetric synthesis, especially methods that graft on chirality with little waste. Collaboration between academic and industrial scientists supports making these breakthroughs reach production plants faster.
Toxicity Research
Toxicological studies point to skin and eye irritation at low exposure, with respiratory impacts for those handling vapors routinely. Chronic exposure can see systemic impacts, drawing on parallels from other halogenated esters. Animal toxicity data guide workplace limits. Researchers seek out ways to minimize waste streams and promote personal protective equipment. Wastewater treatment plants flag this compound and its derivatives for monitoring. Environmental persistence sits in the moderate range, but industrial users must track and report releases under chemical safety regulations. Ongoing reviews suggest cautious optimism as engineering controls and training pull exposure risks lower.
Future Prospects
Looking ahead, the molecule finds itself on a path with higher-value applications, like custom chemical libraries for advanced medicines or tailored agriculture chemicals that use less active ingredient per sprayed acre. Technologies like flow chemistry and smarter sensors help bring production to a lower carbon, lower waste space—something the industry both wants and, increasingly, demands. My experience working alongside manufacturing engineers shows a clear pull toward greener processes and renewable feedstocks. People want safer, cheaper ways to make core intermediates, and research into new catalysts with selective bromination steps grows each year. The drive to minimize environmental impact doesn’t slow down, and the world of ethyl 2-bromopropionate chemistry keeps growing as a result.
A Closer Look at Ethyl 2-Bromopropionate
Ethyl 2-bromopropionate turns up in chemistry labs more often than in household discussions, but its role reaches far beyond a shelf of reagents. The compound carries a simple purpose: it acts as a building block. In organic synthesis, chemists lean on these basic pieces to design more complex molecules.
Making Molecules for Medicine
Pharmaceutical researchers work under tight deadlines to discover new treatments. Ethyl 2-bromopropionate steps in as a useful starting point for creating molecules that go into drugs. Picture the assembly line: a scientist attaches the ethyl 2-bromopropionate to another compound, triggers a reaction, then tweaks the result until the desired product appears. The bromine atom in this compound stands ready for chemical reactions, especially for forming carbon-carbon and carbon-heteroatom bonds, which are the backbone of many medicine-related molecules. Chemists who work on antibiotics or antivirals often need this sort of chemical hook because it makes new structures possible.
Helping Crop Science
Farmers rely on new pesticides and herbicides to keep crops healthy and yields high. Agricultural chemists look for ways to target specific pests without harming the plants or the people who eventually eat them. Ethyl 2-bromopropionate helps them build and test a variety of candidate compounds. Again, its bromine group makes it reactive, which means it can combine with other pieces to develop potent, selective agents. Over the years, this compound has played a hidden role in the innovation pipeline for agricultural chemistry.
Producing Flavors and Fragrances
Odd as it sounds, ethyl 2-bromopropionate sometimes plays a role in making flavors and perfumes. Not because it's tasty or aromatic itself—the compound actually smells sharp and unpleasant—but because it helps chemists make esters and other organic compounds that go into fragrances. Practical experience in a flavor lab shows how tiny changes in a molecule can turn bland chemicals into something that tastes like pineapple or smells like jasmine. Ethyl 2-bromopropionate helps make those changes happen in the lab, then disappears—used up in the process.
Risks and Responsible Use
This compound doesn't belong outside of careful supervision. Like many lab chemicals, ethyl 2-bromopropionate needs safe handling. Direct contact irritates skin and eyes, and inhaling vapors can hurt the lungs. Labs with good track records use gloves, goggles, ventilation, and strict storage to keep people safe. In professional settings, chemical safety data sheets help workers identify risks and appropriate responses. People at home or in schools shouldn't encounter this compound directly; there are plenty of safer alternatives for general education and day-to-day science work.
Finding Greener Chemistry
Working with halogenated compounds such as ethyl 2-bromopropionate has a downside. Disposal requires care to prevent pollution. Progressive labs review their use of brominated and other halogenated chemicals, seeking greener approaches both for the planet and for safety. Chemists share results and methods that cut down on waste, recover starting materials, and find cleaner routes. My own experience cleaning up after old syntheses reveals the value in these changes, since easier disposal and less hazardous byproducts benefit both lab workers and the wider environment.
What Comes Next?
Ethyl 2-bromopropionate won’t draw headlines, but it quietly underpins important advances in medical, agricultural, and industrial chemistry. By using care in handling and remaining open to green innovation, scientists keep extracting value from this compound, supporting progress that eventually reaches all of us, whether on the farm, in the pharmacy, or at the dinner table.
Getting to Know the Structure
Ethyl 2-bromopropionate has the formula C5H9BrO2. As a chemist, I find it interesting how one simple substitution can create a whole new compound with its own behavior. This molecule starts with the propionate backbone, swaps a hydrogen for a bromine on the second carbon, and finishes with an ethyl ester group. That’s the short story written in its formula: five carbons, nine hydrogens, one bromine, and two oxygens.
Unlocking Its Role in Synthesis
Ethyl 2-bromopropionate doesn't just collect dust on lab shelves. It pops up in organic labs worldwide, used for building more complex molecules through reactions like alkylation and nucleophilic substitution. I remember once using it to set up a synthesis for a project involving chiral intermediates. The bromine acts like a flag sticking out, tempting other chemicals to interact in just the right way. In pharmaceutical and agrochemical research, this functionality is key for assembling specialty compounds efficiently.
Chemical Properties and Real-World Use
This molecule’s formula reveals clues about its personality. The ethyl ester moiety gives some volatility, so it tends to waft off if left open too long. The bromine atom brings in some heft and reactivity, making it much more electrophilic than a simple propionate. Reactions involving ethyl 2-bromopropionate generally happen smoothly under mild conditions—it practically invites changes to happen to that reactive brominated carbon.
Researchers and industry professionals count on materials like this to streamline processes. For example, creating pharmaceutical intermediates can stretch across many steps, but a reactive compound like ethyl 2-bromopropionate speeds up those early transformations. Safety needs attention, though. Bromine-containing compounds deserve respect; exposure can cause irritation, and proper ventilation matters in any lab or production setting.
Why the Formula Matters
Recognizing the formula—C5H9BrO2—helps eliminate confusion with close cousins like methyl bromopropionate and other esters. I’ve seen how a mix-up between similar compounds leads to costly delays, especially in pilot-scale production or academic research. Knowledge here spells efficiency and safety. That’s why I always double-check labels and SDS before weighing out a reagent. In many jobs, that simple habit saves both time and trouble.
Safer Handling and Smart Choices
Staff training matters just as much as chemical insight. Lab newcomers sometimes underestimate chemicals with familiar-sounding names. Teaching how to interpret a formula, spot potential hazards, and pick the right protective gear prevents accidents. In my experience, open discussion and clear procedures help teams get comfortable while staying safe.
Room for Improvement
Trying to replace halogenated reagents like this with greener counterparts remains a challenge. Green chemistry groups are on the hunt for alternatives that deliver similar reactivity without bromine or other problematic elements. Until a breakthrough comes, care in storage, transport, and waste handling goes a long way toward minimizing environmental impact.
Every detail in a formula like C5H9BrO2 tells a story about potential, risk, and opportunity. In the hands of careful chemists and well-trained industry workers, the risks shrink and the benefits shine through.
Why Proper Storage Counts
Chemicals like Ethyl 2-Bromopropionate seem harmless when sealed in the lab or warehouse, but nobody should underestimate the risks. I’ve seen what can go wrong if storage gets sloppy: containers bulge, labels fade, fumes escape, regulators come knocking. Careless storage can turn a useful compound into a hazard, both for people and the environment around us.
The Basics: Keep it Cool, Keep it Dry
Heat kicks up trouble. Keep Ethyl 2-Bromopropionate in a cool spot, away from direct sunlight and away from heat sources like steam pipes or radiators. Temperature shifts can spur unpredictable chemical reactions, even slow ones that go unnoticed until there’s a problem. A dedicated chemical storage room with solid ventilation usually outpaces a cluttered closet or an old shelf in the back room.
Humidity brings its own headaches. Even if a bottle looks tight, moisture can get in or slip through a crusty cap, eating away at the compound and making it less stable over time. Store this liquid where it stays dry. That cuts down on corrosion, mold, and label smudging that could leave workers guessing about what’s in that bottle.
Forget Fancy Solutions—Stick with Simplicity and Safety
Glass containers, not plastic jugs, offer the safest bet. Ethyl 2-Bromopropionate reacts with certain plastics, forming peroxides or leaching impurities into the contents. Screw-top glass bottles with clear hazard labels give people a fighting chance if there’s a spill or a question about what’s inside. Never use metal containers, even if they look sturdy; this chemical will chew through most metals thanks to its corrosive and reactive profile.
Always seal the lid tight after every use. Vapors aren’t just a respiratory risk, but can corrode shelving, eat paint, or trigger alarms if they escape into the storage area. Workers in labs and chemical storage areas deserve reliable ventilation, not because it’s a regulation, but because the odd whiff can mean hours of headache or worse.
Controlling the Surroundings
Storing Ethyl 2-Bromopropionate near oxidizers or acids can end in disaster. This liquid reacts with strong bases and strong oxidizing agents; separating storage areas by chemical compatibility keeps things simple and lower-risk. Color-coded shelves or storage cabinets help, but workers also need readable inventory lists so everyone sees what they’re dealing with at a glance.
Some workplaces get lazy about regular inspections. Trust me, skipping weekly checks builds up trouble. Scheduled reviews spot cracked bottles, faded labels, or shelf grime before they invite bigger problems. Inventory logs matter for the same reason. Even in small labs, it’s too easy to lose track and let a chemical go stale.
Personal Experience: Why It’s Worth the Effort
I once worked in a lab that tried to save floor space by bunching chemicals close together. One afternoon, a bottle of Ethyl 2-Bromopropionate shared a shelf with a leaky acid container. The resulting cleanup cost us a day’s work and triggered an investigation that almost lost us our certifications. Since then, I keep chemical safety simple: good containers, cool dry air, solid segregation, clear labeling, and routine checks. It’s not rocket science, but it keeps people safe and saves on costly mistakes down the road.
Better Solutions for Chemical Safety
Invest in spill kits and updated Material Safety Data Sheets (SDS) near the storage zone. Make sure everyone—the new hire, the seasoned tech, the night cleaner—knows emergency contacts and has seen a training session or video on safe chemical handling. All that up-front effort pays off with fewer accidents, smoother audits, and peace of mind at the end of the workday.
A Walk into the Chemical’s Risks
Ethyl 2-bromopropionate pops up in conversation usually because it shows up in labs and factories, not in your average home. For chemists, its uses are clear—it helps put together bigger molecules and crops up in research, especially in pharmaceutical synthesis. Anyone with some chemistry background knows that a lot of useful lab chemicals come with their share of dangers. So does this one pose a real threat?
From Lab Experience to Real-World Concern
During graduate school, I spent months running organic reactions. Many involve compounds a regular person would never touch without gloves, goggles, and a fume hood. Ethyl 2-bromopropionate never sat on my shelf, but cousins in the bromoester family did. Their sharp, sometimes sweet odor made it clear—nobody wanted to get too close. The bromine atom alone signals a warning. Most brominated organics irritate eyes, skin, and airways, with some causing lasting damage if exposure happens enough times.
Looking into the safety data, ethyl 2-bromopropionate can harm not only with direct splashes but also through its vapors. Studies point to burns or rashes when it meets skin, irritation if it lands in the eyes, and breathing trouble if someone smells it for too long. Some research on similar chemicals found that wearing gloves, goggles, and using ventilation meant accidents stayed rare, but untrained handling led to emergencies. Brominated compounds sometimes affect the nervous system too. Workers exposed over weeks—without the right protection—showed tremors, nausea, and headaches. These stories aren’t just in textbooks; old timers in the chemical trade will talk your ear off about burns and poison scares before rules got strict.
Balancing Practicality and Protection
Companies and universities run through long checklists before anyone pops the cap on a bottle of ethyl 2-bromopropionate. They track spills, log who used what, and insist students take training. From my own desk, I know that these rules take time and patience, but skipping steps brings real trouble. Chemical hygiene officers sometimes sound over-cautious, but after seeing hospital trips from careless handling, I understand their worry. In my lab days, the fume hood’s glass barrier felt reassuring—nothing quite like the sting of a chemical splash to make you respect those layers of defense.
Solutions: Clear Rules, Better Training, and Layered Defenses
Not every workplace puts safety first. Smaller businesses and colleges sometimes scrape by with old gloves, broken exhaust fans, and poorly translated safety sheets. Culture makes a difference. Supervisors must walk the talk—reminding everyone that short cuts aren’t worth it. It’s not enough to post safety sheets in a binder; people need to recognize that chemicals such as ethyl 2-bromopropionate can harm for real. Experiences shared during training sessions help the lessons stick. I once heard a story from a technician who didn’t know about the risk and paid with a nasty rash and weeks off work. That was a lesson better learned from his story than repeating his mistake.
Research into safer handling and substitution matters too. Scientists keep looking for alternative chemicals that deliver the same results with less risk. In some cases, newer green chemistry techniques cut down on brominated compounds altogether. For now, anyone in a lab should remember that safety gear, regular training, and honest reporting form the best defense. Clear labeling and storage rules help make sure nobody grabs the wrong bottle or makes a dangerous mix.
Knowledge, Culture, and Responsibility
The facts point to a simple truth: ethyl 2-bromopropionate brings more risk than most household chemicals. For many workers, diligence and respect for best practices make all the difference. We owe it to each other to keep risk down, not just in big companies, but anywhere these chemicals show up. That lesson stays with me, and I hope it will land with anyone handling something as tricky as ethyl 2-bromopropionate.
Understanding This Liquid’s Real Personality
Working in chemistry labs means spending a lot of time getting to know chemicals on a very personal level. Chemicals like Ethyl 2-Bromopropionate don’t make headlines, but they show up in plenty of places—from pharmaceutical ingredients to unique reactions in research. Anyone working with it quickly notices it’s not just another bottle on the shelf.
Physical Details That Stand Out
Ethyl 2-Bromopropionate shows up as a clear, colorless to pale yellow liquid. In my own experience, that faint yellow hue sometimes creeps in if it’s been stored a bit too long or hasn’t been kept tightly sealed. It gives off a sweetish, pungent scent—it’s distinct but not overpowering. The smell might stick to your gloves after handling, so there’s no mistaking what you’re working with.
On the scale, its weight surprises some folks. The density lands around 1.41 g/cm³ at room temperature, which helps explain why it feels heavier when pouring compared to, say, regular ethyl acetate or even water. Spills move slower, and cleanup feels slightly less frantic, but splashes linger longer too.
Boiling and Melting Points
Boiling this compound brings the typical safety warnings to mind. It boils at roughly 148°C, which means handling requires more than a hot plate—it needs careful temperature monitoring and good ventilation. In colder storage, the freezing point hangs down near -65°C. It stays liquid in a wide range of laboratory or industrial settings, making it easy to keep on hand or ship to other labs.
Solubility and Reactivity
Anyone who’s mixed this in a flask knows it doesn’t get along with water. It’s only slightly soluble, forming two layers instead of one. It dissolves well in common organic solvents like ether, chloroform, and alcohol. This behavior shapes how it’s used in syntheses. Purification by aqueous workups means paying close attention to phase boundaries.
Chemists know halogenated esters like this one tend to be more reactive. That bromine atom doesn’t just sit there; it helps form new carbon-carbon bonds and swaps out for different chemical groups with ease. Over many reactions, I’ve seen it behave as a good alkylating agent, adding new sections to growing molecules. The reactivity means double gloves and quick access to the fume hood are a must.
Why This All Matters
With so many chemicals on the market, knowing these physical properties isn’t just lab trivia. If ethyl 2-bromopropionate didn’t have this mix of volatility and decent solubility in organics, certain pharmaceutical routes would get a lot more complicated. Mistakes in estimating density lead to faulty yields. Overheating during distillation can risk breakdown or exposure. Small changes in color warn about possible decomposition, saving projects and equipment from bigger disasters.
A simple web search gives fact sheets, but day-to-day experience cements the importance of these details. Handling this liquid means relying on memory, notes scribbled on labels, and trust in the gloves and hoods that keep us safe. A little more knowledge always leads to a smarter, safer lab environment—and better outcomes for the people using these chemicals on real projects.