2-Cyanophenol: An In-Depth Commentary
Historical Development
Interest in 2-cyanophenol traces back to the rise of organic chemistry in the early 20th century. Researchers hunting for effective intermediates for dye and pharmaceutical synthesis discovered its quinonoid backbone offered more than just structural novelty. Industrial laboratories moved from simple curiosity to commercial-scale production, often following the pace of the synthetic dye fields blossoming in Europe. Companies valued it for its versatile aromatic ring, which opened many possibilities. Over decades, production shifted from laborious, small-batch operations to efficient, continuous flow procedures. The needs of war, peace, and industry shaped how 2-cyanophenol found a place in chemical supply chains. By the 1950s, standards for chemical purity and safe handling pushed researchers to refine its synthesis, storage, and labeling practices.
Product Overview
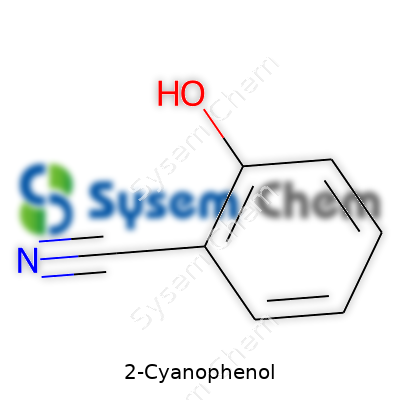
2-Cyanophenol stands out as an aromatic organic compound, essentially a phenol molecule where one hydrogen atom on the benzene ring gives way to a cyano group at the ortho position. Its significance rests not in household recognition but in how many specialty chemicals rely on it mid-synthesis. Dye makers, pharmaceutical formulators, and polymer scientists all reach for 2-cyanophenol when simpler compounds fall short of delivering the functionality they need. While not as ubiquitous as phenol or benzonitrile, its reactivity and manageable toxicity support uses in both academic research and industry. I’ve seen it serve as a critical link connecting basic aromatic chemistry to commercial-scale products, especially in custom fine chemicals sectors that supply everything from pigments to drugs.
Physical & Chemical Properties
2-Cyanophenol usually shows up as beige to pale brown crystals or powder, a bit less pure when stored improperly or handled without care. With a melting point near 110°C and a boiling point between 290–300°C, the compound fares well in most typical laboratory environments, neither evaporating too easily nor breaking down under moderate heat. Its most striking feature is the interplay between the acidic hydroxyl group and the electron-withdrawing nitrile, giving the molecule a balance of nucleophilicity and electrophilicity. This lets chemists gently nudge the compound along a variety of synthetic paths. Slight-soluble in water, it dissolves smoothly in organic solvents like ether or ethanol, which makes it easy to integrate into chemical reactions without too much fuss.
Technical Specifications & Labeling
Most suppliers offer grades of 2-cyanophenol with purity levels above 98 percent, reported by gas chromatography or high-performance liquid chromatography. Certification sheets spell out residual solvents, heavy metal content, and moisture, satisfying regulatory or end-user demands. Bottles carry hazard pictograms according to GHS rules, along with recommended protective measures. Storage calls for airtight containers, cool and well-ventilated areas, and clear identification on all paperwork. Even small lapses—like ambiguous batch codes or smudged expiration dates—can trip up audits or quality checks. Safety isn’t theoretical here: A misplaced or wrongly labeled bottle once led a group I worked with to discover, after hours, that an intended catalyst was instead a similar-looking cousin, leading to a failed reaction and wasted resources.
Preparation Method
Laboratories and industry typically synthesize 2-cyanophenol through nucleophilic substitution of ortho-halophenols with copper-catalyzed cyanation. Halide selection shapes reaction conditions; 2-chlorophenol can work, but higher yields come from the iodo variant. A shift towards less toxic reagents and recyclable solvents has shaped modern protocols. Scale-up from bench to kilo lab demands sharper temperature control and more reliable metering of cyanide sources, as free hydrogen cyanide represents a pronounced safety risk. Alternative, greener avenues continue to attract research grants, seeking catalysts that minimize side products or byproducts toxic to aquatic environments. Teams must routinely calibrate their equipment and ventilate properly; just a few lapses can lead to ghostly blue stains or faint almond odors signaling potential danger.
Chemical Reactions & Modifications
2-Cyanophenol pairs its aromatic reactivity with tricky but useful side groups. The phenol ring supports electrophilic substitutions, while the ortho-cyano group activates the molecule toward nucleophilic additions. Skilled chemists turn these features to advantage, crafting substituted quinolines, isoxazoles, or benzoxazoles through condensation, cyclization, or reduction. Hydrogenation of the nitrile yields 2-aminophenol under mild conditions. Custom tailors in research labs often tweak the hydroxyl or cyano end to suit requirements for target drugs, dyes, or even light-absorbing polymers. The flexibility appeals to those exploring new pigment chemistries or tailoring ligands for transition-metal complexes. Each pathway carries risks of side products, especially if moisture finds its way into a supposedly dry flask.
Synonyms & Product Names
This compound lacks the branding of some specialty chemicals, yet its alternative names—ortho-cyanophenol, 2-hydroxybenzonitrile, or o-hydroxybenzonitrile—appear across catalogs and safety data sheets. While these names seem interchangeable, confusion can crop up in ordering or communication, especially when language barriers or outdated documents enter the mix. I’ve seen procurement delays trace back to a simple mix-up between methyl- and cyano- derivatives, underlining the value of standardized catalog numbers and rigorous checking. Regulatory filings and customs declarations depend on precise naming, so oversight at this level causes headaches down the line.
Safety & Operational Standards
Work with 2-cyanophenol draws firm lines—use gloves, eye protection, and good ventilation as a rule, not an afterthought. Both the hydroxyl and nitrile functions raise concern: inhalation or prolonged skin contact brings acute irritation, headache, or worse, possible sensitization. Cyanide chemistry carries grim historical baggage, so protocols outline clear procedures for spills and exposure. Anyone working routinely with cyanophenol must know how to recognize acute symptoms and act, not just rely on a supervisor or first-aid chart. Facilities store only what is needed, segregated from acids and oxidants. Waste management matters, too: untreated effluents can harm biota far beyond the plant gates. Inspections and drills reinforce not just compliance but muscle memory for safe handling.
Application Area
Few people outside specialty chemistry circles recognize the name, yet 2-cyanophenol threads through daily life by way of its downstream products. Dye manufacturers use it to anchor colorfastness in synthetic fabric dyes. Pharmaceutical labs exploit its phenolic and nitrile moieties as scaffolds for coupling reactions during drug candidate synthesis. Agrochemicals benefit as well; some herbicides and fungicides trace critical steps to cyanophenol chemistry. Polymer researchers incorporate it when tuning optical or electronic properties. I’ve seen research groups reach for 2-cyanophenol almost instinctively when facing the stubborn synthesis of nitrogen-containing ring systems, knowing that the extra electron-withdrawing punch can nudge reactions where other starting materials fail.
Research & Development
The hunt for more efficient, cleaner cyanation methods keeps research vibrant. Catalysts—copper, palladium, nickel—each bring their own quirks and benefits to improving yields and reducing hazardous waste. High-throughput screening now pinpoints reaction conditions in days rather than weeks. As environmental regulations force a rethink of acceptable byproducts, R&D teams search for greener solvents and alternative reagents to reduce risk. I have seen innovation thrive in university-industry partnerships, drawing on government grants and real-world constraints. Patents spike each year for derivatives of 2-cyanophenol as starting points for entirely new product lines in electronics, therapeutics, and advanced coatings.
Toxicity Research
Concerns about the biological effects of cyanophenol derivatives date back decades. Bioassays demonstrate acute toxicity to aquatic organisms at low micromolar concentrations. Membrane permeability makes skin contact particularly troublesome. Chronic exposure risks remain less well-defined, partly due to limited long-term animal studies outside the most common derivatives. Regulators, drawing on European REACH and US EPA data, set occupational exposure limits to guard against both acute and latent harm. Many applications have shifted to closed-system manufacturing or remote processing just to keep airborne residue within safe bounds. Teams rely heavily on up-to-date hazard assessments and monitoring; safety slips erode not only worker health but also erode the trust needed to keep valuable chemistry alive.
Future Prospects
The compound’s flexibility almost guarantees a future in fine chemical development. As more industrial buyers look for greener synthesis, lower toxicity, and tighter regulatory compliance, 2-cyanophenol’s chemistry stands ready for adaptation. I expect innovations in continuous-flow reactors will shrink risk and cut production costs. More sustainable cyanation reactions seem poised to reduce the headaches tied to cyanide handling, opening doors to smaller manufacturers and new research groups. Outside traditional uses, the surge in specialty materials—organic electronics, responsive coatings, biomedical polymers—points to even wider utility. Demand from drug discovery and specialty pigment sectors won’t disappear, and every new regulatory tightening nudges supply chains toward higher purity, safer packaging, and tighter traceability. Junior chemists cutting their teeth on aromatic substitution will keep 2-cyanophenol in their toolkits, pairing curiosity with value as the field continues to evolve.
Understanding the Role of 2-Cyanophenol
Most people never hear about 2-Cyanophenol unless they work in a lab or a chemical plant. This isn’t stuff you find on a grocery store shelf or used around the house. Still, compounds like this end up touching lives more than you’d think. I remember handling it during a college internship at a specialty chemicals facility. Even with safety gloves on, the distinctive smell stayed with me until the end of my shift.
Core Uses in Pharma and Fine Chemicals
The world of pharmaceuticals leans on 2-Cyanophenol for its knack at building complex molecules. Chemists often see it as a versatile starting point. Its cyano group and phenolic structure allow for plenty of chemistry tricks—think about stringing together bigger molecules. I learned that certain psychiatric drugs and anti-inflammatory ingredients stem from these sorts of blocks. Some companies use it as a precursor for drugs aimed at neurological disorders or pain relief. Without such intermediates, making advanced medicine gets tougher and more expensive.
Colorants and Dyes: Bright Hues, Subtle Chemistry
The colors on fabrics, the deep blacks in printer ink, and even some food-safe colorings have roots in this compound. Dye production relies on aromatic molecules just like 2-Cyanophenol. Its chemical structure lets it react easily, forming color-rich molecules that latch onto fibers. Over the years, textile and ink factories have looked for ways to get longer-lasting, better-penetrating color, and chemistry like this helps. From what I’ve seen at trade shows, small tweaks in such starting products give rise to a whole spectrum of colors.
Advancing Agriculture: Crop Protection
Farming depends on chemical innovation as much as steel and soil. Herbicides and pesticides sometimes rely on building blocks like 2-Cyanophenol. Certain plant protectants use these aromatic pieces for their effectiveness against bugs or weeds. Not every farm wants to spray heavy toxins, so researchers keep looking for safer options that break down faster. Over the last decade, safer derivatives have made headway, many starting with the cyano-phenol building block.
Specialty Materials, Resins, and Beyond
Industries crave materials that can resist heat or chemical attack. Labs mix 2-Cyanophenol into specialty polymers and resins. These resins end up in electronics, high-end coatings, or adhesives. In a world with so many wireless gadgets, demand keeps growing for safer, stronger plastics. My neighbor, who designs circuit boards, explained that certain synthetic varnishes owe their toughness to molecules built from compounds like this.
Hurdles and Hope for Safer Production
Handling 2-Cyanophenol puts safety right up front. It can irritate skin or eyes, needing gloves and goggles. Factories need to keep fumes down and waste under control. Some plants have switched to better ventilation and faster clean-up to lower risks, but accidents still happen. I’ve met safety trainers who believe knowledge beats fear—they run hands-on training so workers don’t make mistakes with these compounds.
Moving Toward Greener Chemistry
The chemical world wants to cut back on waste and make production cycles easier on the environment. Greener alternatives keep popping up—like high-yield reactions needing less solvent, or recycling more leftover materials. Some chemists are testing plant-based routes instead of old petroleum paths. As more industries demand safer chemicals, the push keeps growing to refine how starting blocks like 2-Cyanophenol are made and handled.
Working With Chemicals: Lessons Learned in the Lab and Beyond
Long days in industrial labs have taught me that no shortcut saves time when dealing with chemicals like 2-cyanophenol. It’s not just the formal training—over the years, personal experiences with skin rashes and close calls with volatile fumes underline how important everyday vigilance is. Glossing over small details almost always leads to problems down the line. The sharp, almost pungent smell of 2-cyanophenol lingers in memory long after the workday ends, serving as a reminder to keep good habits up front.
The Risks of 2-Cyanophenol
Inhalation causes headaches, coughing, and irritation of throat or lungs. Even small splashes on skin invite trouble—burning, redness, sometimes more severe reactions. Contact with eyes risks serious injury. It only takes a light breeze to lift dust, spreading risk well beyond the original container. Extended or repeated exposures raise odds of more profound health problems.
Personal Protection Always Comes First
Nobody enjoys donning gear in summer, but skipping goggles or gloves never pays off. Nitrile gloves hold up better than latex or vinyl with 2-cyanophenol. Tucked sleeves, lab coat buttoned, and a solid pair of goggles that wrap around the eyes close off weak spots. In professional settings, respirators with organic vapor cartridges protect from inhaling what’s in the air, especially during transfers or spills. Sturdy shoes—not sandals or sneakers—should always be the norm.
Ventilation: Not Just a Box to Tick
Fume hoods and local exhaust make all the difference. I’ve seen too many rooms fill up quick with invisible vapors because someone underestimated diffusion. Good setups keep air moving away from breathing zones. Even in small shops, cracked windows alone don’t provide enough airflow. Regular checks and maintained fans help keep accidental exposures in check.
Handling and Storage: Simple Steps Pay Off
Storing 2-cyanophenol away from acids, oxidizers, and direct sunlight avoids chemical surprises. Piling materials or leaving lids loose caused mix-ups in more than one facility I’ve visited. Colored, labeled containers guard against confusion and let emergency responders spot trouble quickly. Smaller quantities reduce risk—the less you handle, the less that can go wrong.
Emergency Measures: Preparedness Beats Reaction
Plenty of people freeze up during emergencies. Eyewash stations and safety showers need to be kept unblocked and tested each month. In case of skin or eye contact, immediate copious rinsing with water makes a huge difference. Spills demand absorbent materials—not just paper towels. Spill kits with neutralizing agents belong near work areas. Most important, make sure that phone numbers for medical help and poison control are posted nearby.
Training and Habit
Reading the SDS and keeping it within arm’s reach is not just bureaucratic ritual. Regular training, hands-on drills, and open conversations about near-misses build a sense of responsibility. Sometimes, the lessons come from others’ mistakes. Sharing stories about real accidents helps shift complacency and sparks a change in attitude across the team.
Room for Improvement
Encouraging a culture where people report and learn from small mistakes—without shame—pays off. Good lighting, updated equipment, and better training materials all help. Supervisors who set a visible example and value feedback from anyone in the room improve compliance in ways rules alone never manage.
References: Centers for Disease Control and Prevention (CDC), National Institute for Occupational Safety and Health (NIOSH), and personal experience from years in chemical process labs.Getting to Know 2-Cyanophenol
2-Cyanophenol stands out as a benzene ring carrying both a cyano group and a hydroxy group. You’ll spot the cyano group (-C≡N) at position two on the ring, right next to where you’d find the hydroxy group (-OH). From a structural point of view, imagine a benzene ring with the -OH group at position one, and the -C≡N kicked in directly next to it at position two. Its formula, C7H5NO, gives away the mixture of carbon, hydrogen, nitrogen, and oxygen at play. This arrangement isn’t an accident; it gives the compound unique behavior and value.
Why Structure Tells a Bigger Story
The power of 2-Cyanophenol’s structure goes beyond chemistry class. This pairing of cyano and hydroxy groups isn’t just academic. It means the molecule can hook up with different reagents in organic synthesis, especially in the pharmaceutical and pigment industries. The electron-withdrawing cyano group tugs on the electrons in the ring, and the hydroxy group’s reactivity gets influenced by the neighborhood effect. The unique positioning changes acidity and makes it easier to modify in lab work. These tweaks let chemists target 2-Cyanophenol when building more complex compounds. Such details shift theory into practical gains.
Real-World Impact
With its twin functional groups, 2-Cyanophenol becomes essential for creating specialty chemicals and advanced materials. Its blueprint drives the search for new pharmaceuticals and agrochemicals. Research teams frequently use it as an early building block because its structure supports further customization. While some lab chemicals seem distant from daily life, 2-Cyanophenol takes on jobs that touch eventual real-world needs—think about dyes in clothing or components for electronics. Even tougher synthetic challenges in making new drugs come one step closer because chemists rely on structures like this as a base for more intricate molecules.
Addressing Challenges Around Handling and Use
Experience with aromatic compounds like 2-Cyanophenol underscores the need for safe and informed handling. The cyano group’s reactivity brings not only value but also some risks. In my own work, gloves and fume hoods always stand as standard safety measures. Mistakes stem less from a molecule itself than from rushed or careless work. As research circles push deeper into using advanced intermediates, up-to-date safety sheets and careful risk assessments become more than just paperwork—they help prevent dangerous exposures before they begin. The right structure doesn’t just power innovation; it demands respect and responsibility.
Shaping Solutions and Future Directions
One clear avenue for progress involves supporting green chemistry when working with 2-Cyanophenol and similar compounds. Labs have shifted toward milder solvents and more sustainable practices by necessity, cutting waste and lowering exposure to hazardous byproducts. Integrating greener processes allows organizations to keep moving forward while watching out for their workers and the surrounding environment. Cross-disciplinary teams, from toxicologists to engineers, keep the conversation balanced: how to unlock the molecule’s strengths while minimizing risk.
Takeaways Built on Experience and Fact
By paying attention to the specifics of 2-Cyanophenol’s structure—down to exactly where each group attaches—chemists and researchers gain flexibility for synthesis and application. Pairing technical know-how with hands-on safety and environmental awareness, both in labs and industrial settings, helps the field push ahead. The structure of 2-Cyanophenol isn’t just a map on a page; it opens doors for new science, safer methods, and direct outcomes the public actually feels.
The Real Risks with 2-Cyanophenol
2-Cyanophenol isn't something you want piled up on a shelf next to the snacks in a lab. I’ve seen what a little misplaced care can do with chemicals near this level, from corroded shelving to strong, irritating odors that won’t leave your lungs or workspace for hours. Even before diving into guidelines, experience around chemical storage tells me that even one shortcut can lead to a long clean-up, if not something worse.
This purple-tinged chemical, often used in dyes and pharmaceuticals, gives off vapor that can irritate the eyes, skin, and respiratory tract. Breathing in even small amounts makes for a rough afternoon. Long exposure or accidental spillage means double trouble. Handling dangerous chemicals without the right respect leads to lifelong lessons—usually the hard way. I’ve seen it turn a normally careful research assistant into a regular at the campus health office.
Temperature and Light Aren’t Just Afterthoughts
I once worked in a lab where we stored materials just about anywhere with shelf space. Sun through windows turned the storeroom into a sauna. For 2-cyanophenol, warm rooms aren’t harmless. The stuff can decompose quicker under heat, sending off toxic fumes. For chemical safety, a dedicated storage cabinet with temperature controls feels less like a luxury, more like an insurance policy against mistakes. Keeping it cool—typically well below room temperature—shrinks the risk of accidental vapor release and keeps reactivity in check.
Bright light isn’t much better. Prolonged exposure to UV rays can degrade the compound and increase the odds that you’re stuck with byproducts you never intended to handle. Storing 2-cyanophenol in opaque or amber bottles, inside a dark cabinet, keeps it as stable as possible and reduces chances of accidental exposure. It’s a practice that’s paid off across plenty of labs and one I'm not likely to skip.
Material Compatibility and Containment
2-cyanophenol reacts with strong oxidizers and acids, even in tiny splashes. I once watched a spill that crept down behind a shelf. The mix with leftover acid turned an afternoon into a fire drill. Plastic containers often won’t hold up if there’s any chemical attack. Glass bottles with screw caps, fitted tightly and labeled clearly, keep things controlled and predictable. Polyethylene liners give a little more confidence, adding another barrier between the cyanophenol and the outside world.
Secondary containment trays are just common sense. If a bottle leaks or breaks, everything stays together instead of dripping down to where people store their shoes. Storing incompatible chemicals apart from each other isn’t just a checklist item. It’s something to take personally—cross-contamination shortens both experiments and careers.
Ventilation and Accountability
I’ve worked in small research spaces and big industrial settings, and both share one critical truth: enclosed cabinets with good ventilation prevent vapor buildup. Chemical fumes creep through the smallest gaps and can stick around long after anyone remembers why their eyes sting. Storage closets with independent extraction fans make a real difference. Good signage and up-to-date chemical inventories, with trained staff who know what’s inside, avoid confusion before anyone reaches for the wrong bottle.
Why This Matters
Sloppy storage choices with substances like 2-cyanophenol only feel convenient in the short term. The cost of poor storage—ruined equipment, workplace injuries, and regulatory headaches—outweighs any time saved. Choosing secure, cool, dark, and ventilated storage means protecting the people who work in these spaces as much as the research. It’s not just about rules or paperwork; it’s about making sure everyone gets safely home at the end of the day.
Understanding the Basics
As someone who’s dealt with a fair share of organic compounds in the lab, I can’t help but take a close look at 2-Cyanophenol. At first glance, this compound with the chemical formula C7H5NO might just seem like another member of the phenol family, but that extra nitrile group on the second position sets it apart and brings some quirks to the table.
Appearance and Texture
2-Cyanophenol normally forms as a solid at room temperature. Many phenol-based compounds lean toward crystalline or powdery textures, and this one fits the bill. Its color often runs from off-white to a faint yellow. This isn’t the kind of color that jumps out at you, but under the right light, there’s no mistaking it for a perfectly pure white compound. If you’ve handled phenolic or aromatic powders, you’ll recognize the dry, fine feel that 2-Cyanophenol offers—something that doesn’t clump or cake easily.
Melting and Boiling Points
Temperature change tells a lot about any chemical, and 2-Cyanophenol doesn’t disappoint. It melts around 114–116°C, which lines up with the presence of both an aromatic ring and a nitrile group. Heat it beyond this range and expect it to start boiling near 277°C. In my experience, these temperature thresholds contribute to safe handling: the melting point is well above room temperature, so accidental spills stay solid, and it won’t start boiling unless someone’s pushing things well past typical benchtop conditions.
Solubility Profile
Solubility carries weight for anyone trying to use or manage 2-Cyanophenol. It dissolves moderately in water, but not in the amounts that ethanol or acetonitrile might manage. Its phenolic OH group likes forming hydrogen bonds, so you get some play with water—enough to notice a slight dissolve, especially if you warm things up. On the flip side, organic solvents like ether and acetone treat it much more kindly. You’ll see 2-Cyanophenol dissolve faster in these settings, making purification or reaction setups less of a hassle.
Odor and Volatility
Anyone cracking open a vial of this compound for the first time will catch a whiff of something subtly aromatic. The smell isn’t as biting as plain phenol, but there’s a certain sharpness that signals the presence of that nitrile addition. Its volatility sits firmly where you would expect for something with this kind of structure—stable under normal air, but far from totally inert if exposed to open air for extended periods. The vapor won’t fill the whole room, which matters for anyone working in confined spaces.
Safety and Handling Concerns
Most phenolic compounds demand respect, and this one is no different. Exposure can lead to irritation, especially if allowed to contact the skin or eyes. Lab handling should always include gloves and eye protection. Dust can be a problem if stirred or transferred roughly, but the comparatively high melting point keeps things neat compared to lower-melting organics. Short-term exposure to small amounts—common in many synthesis routines—rarely causes immediate problems, but chronic or repeated contact should be avoided.
Density and Practical Use
With a density of about 1.2 g/cm³, you’ll notice this material doesn’t float or puff up easily when measured. It’s easy to weigh or transfer on a balance without worrying about static or airborne dispersion. This helps both in precise dosing for chemical reactions and when preparing crystalline samples for further study.
Reflections on Real-World Significance
Grasping these attributes doesn’t just round out a mental image of the compound—it helps keep people safe and ensures the chemistry goes according to plan. Synthesizing, purifying, or analyzing 2-Cyanophenol, I’ve found that these basic traits guide everything from equipment choice to waste disposal. Chemistry isn’t just theory; it’s about staying alert to the fine print, and the physical properties of 2-Cyanophenol show just how much those details can matter.