2-Bromopropionyl Bromide: A Deep-Dive into Its Role, Risks, and Reach
Historical Development
Chemistry in the early 20th century saw a flood of innovation, and 2-bromopropionyl bromide emerged during a rush to get better intermediates for pharmaceuticals and novel materials. Early patents hinted at using brominated acyl halides for rearrangements and substitutions. Lab notebooks from the 1950s show chemists reaching for 2-bromopropionyl bromide when standard acyl bromides struggled with selectivity. Over the decades, as organic synthesis kept growing, it built a reputation among chemists for its versatility, especially where site-specific bromination mattered.
Product Overview
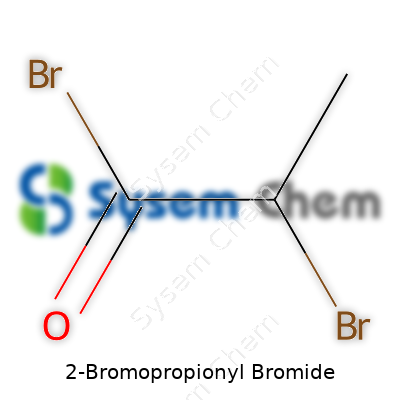
2-Bromopropionyl bromide carries the formula C3H4Br2O and comes across as a clear, colorless to pale yellow liquid with a sharp smell that lingers in the air. Most catalogs put it under specialty acylating agents. The backbone—a propionyl group with two bromines—allows for strong reactivity both at the carbonyl and on the alkyl chain, attracting researchers looking for new ways to build complex molecules. Its supply chain tends to feed mainly pharma, agrochemical, and cutting-edge materials research.
Physical & Chemical Properties
High volatility defines laboratory handling of 2-bromopropionyl bromide. Its boiling point hovers around 120-122°C under atmospheric pressure, and it reacts strongly with water to produce corrosive fumes of hydrobromic acid. The density outpaces water, sitting around 2.0 g/cm³. This compound does not dissolve in water but mixes with organic solvents such as dichloromethane and chloroform. Reactivity comes from both the acyl bromide group—which acylates amines and alcohols briskly—and the bromo-methyl group, which undergoes nucleophilic substitutions. The combination of these sites shapes its chemical behavior in every synthesis.
Technical Specifications & Labeling
Labeling for 2-bromopropionyl bromide includes hazard pictograms for acute toxicity, corrosive action, and environmental risk. Purity often reaches 98% or more, and reputable suppliers offer detailed certificates of analysis covering moisture content, residual solvents, and stabilizer content. Container sizes tend to range from grams for research to multi-kilo batches for pilot plant runs. Safety covers airtight seals and amber glass bottles to block degradation from light, with backup labels describing the need for PPE and handling within fume hoods.
Preparation Method
Commercial production usually begins with 2-bromopropionic acid. Treating this acid with phosphorus tribromide or thionyl chloride in the presence of excess bromine creates 2-bromopropionyl bromide in a straightforward conversion. The crude product, collected via distillation under reduced pressure, requires careful fractionation to remove unreacted halides and side-products. This direct halogenation route keeps costs lower and gives a consistent yield, milestones the chemical industry looks for when scaling up specialty reagents.
Chemical Reactions & Modifications
2-Bromopropionyl bromide earns its keep by acylating amines to give N-bromoalkylated amides—a trick that regular propionyl bromide misses. Researchers use its two reactive sites to introduce brominated side chains into drugs, agrochemicals, and functionalized polymers. Tackling nucleophilic substitution on the side chain opens a pathway for building unusual heterocycles and peptide mimics. Reductive debromination lets chemists strip away bromine after it has served its purpose as a protective or activating group, offering freedom to adjust synthetic plans midstream.
Synonyms & Product Names
Chemical catalogs and research papers often mention alternative names like alpha-bromopropionyl bromide, 2-bromo-1-bromopropanone, or Propanoyl bromide, 2-bromo-. Some legacy documents refer to it as 2-bromopropanoic acid bromide. The CAS number 587-91-1 serves as a unique identifier in trade and compliance documentation, helping avoid confusion with similar brominated compounds.
Safety & Operational Standards
Strong acyl halide reactivity demands rigorous safety routines. Respiratory protection is not just a guideline but essential, since vapors can burn the respiratory tract even at low concentrations. Spills attack skin and eyes, with even short exposure leading to burns or ulceration. Working with 2-bromopropionyl bromide means a fume hood, gloves rated for corrosive acids, and full coverage goggles. Emergency protocols in facilities using this compound need eyewash stations and proper acid-neutralizing materials. Without these defenses, injuries and environmental releases can happen in seconds. Transport rules treat it as a regulated hazardous substance under UN 3265 (Corrosive Liquid, Acidic, Organic, N.O.S.), and disposal goes through high-temperature incineration—no exceptions.
Application Area
2-Bromopropionyl bromide finds itself at home in pharmaceutical labs, where synthesis of antiviral or anticancer candidates requires bromoacyl intermediates for SAR (Structure-Activity Relationship) tweaking. Crop science has picked it for certain herbicide precursor syntheses, and the surfactants sector uses it to craft specialty emulsifiers for demanding formulations. In advanced materials, introducing bromine atoms through this reagent fine-tunes properties in polymers looking for new applications in electronics or membrane science. Each application pushes the envelope on molecular architecture, highlighting just how adaptable this chemical can be when guided by thoughtful design.
Research & Development
Modern R&D efforts focus on replacing more hazardous acylation protocols and moving toward greener synthesis. 2-Bromopropionyl bromide, due to its reactivity and selectivity, offers an alternative to harsher conditions found in chlorinated routes. Researchers test it in high-throughput settings, seeking to map which bioactive scaffolds benefit most from precise bromine introduction and how it speeds up the assembly of complex molecules. Recent papers explore catalysts that could lower temperatures and waste downstream, demonstrating ongoing investment in safer, more sustainable applications.
Toxicity Research
Lab tests show that 2-bromopropionyl bromide causes acute damage to skin, mucous membranes, and respiratory tissues, which aligns with findings for similar brominated acyl halides. Rodent studies reveal lethality at low concentrations, with repeat exposures quickly attacking vital organs. Chronic exposure profiles remain less well studied, but short-term experiments underline the need for strong protections. Regulatory agencies demand restricted access and thorough training because toxicity parallels compounds that have caused severe accidents in the past.
Future Prospects
Tomorrow’s uses for 2-bromopropionyl bromide depend on advances in synthetic methodologies and regulatory shifts toward cleaner chemistry. Pharmaceutical innovation—especially for custom medicines—leaves a market for high-purity, specialty acyl bromides. Pressure grows for suppliers to cut impurities and shrink their carbon footprint, maybe by recycling bromine or coming up with continuous processes that minimize waste. The material science realm, powered by new electronics and membranes, may discover unique uses for tailored brominated building blocks. Green chemistry research will dictate how this compound fits into production pipelines that value both performance and safety. The future belongs to those willing to rethink risks while pushing molecular design into new territory.
A Close Look at the Formula
2-Bromopropionyl bromide carries the formula C3H4Br2O. On paper, that set of letters and numbers might seem unremarkable. Yet for chemists, this formula tells more than the sum of its parts. You get three carbon atoms, four hydrogens, two bromine atoms, and one oxygen. The arrangement matters just as much as the content. In this case, a bromine sits on the second carbon, with a bromine attached as an acyl bromide group.
Why This Formula Matters in the Real World
In lab work, especially in pharmaceuticals and specialty materials, precision is everything. Chemists depend on exact formulas to steer the outcome. A slip up, even at the atomic level, can wreck a project or worse, compromise safety. From my own days in a college organic lab, the wrong halogen in a synthesis led to weeks of wasted effort. Given its formula, 2-bromopropionyl bromide serves as a building block in the creation of more complex molecules—often those used in new drugs and agricultural products.
Hands-On Experience With 2-Bromopropionyl Bromide
Colleagues in research settings often nod knowingly at the mention of 2-bromopropionyl bromide. It’s not a household chemical like vinegar or bleach. Instead, it’s the kind you find in flasks behind glass shields, handled with gloves and careful training. The two bromines give it unique reactivity, making it useful for introducing new functional groups to molecules through a process called acylation. This doesn’t just speed up drug research—it makes reactions that wouldn’t be possible otherwise a real option.
Weighing the Safety Factors
Working with C3H4Br2O brings real risks. Its strong, acrid smell signals the need for good ventilation. Chemical burns and lung irritation are no joke. Proper PPE stops accidents before they start. Labs rely on well-established safety data and protocols, but every user has a duty to keep an updated material safety data sheet within arm’s reach, especially with compounds like this one. I’ve seen firsthand what can happen when a fume hood fails and vapors spread—a fast evacuation, and grateful faces after the all-clear.
Supporting Safe and Responsible Use
Safe storage keeps reactivity and volatility in check. Storing this chemical in a dry, cool area away from acids and moisture prevents dangerous decomposition or explosive scenarios. It always arrives in sealed glass, with secondary containment for transport. Leading labs follow best practices from organizations like OSHA and the European Chemicals Agency, drawing on decades of real-world data to keep people and projects safe.
Looking Toward Better Solutions
Chemistry evolves quickly. Green alternatives and safer analogs are always in demand, especially given the environmental impact of using halogenated reagents. Research focuses on developing new pathways that cut down on toxic waste and limit worker exposure. Strong oversight, clear labeling, and investment in cleaner technologies make a difference, but it all starts with knowing your formula—C3H4Br2O—inside and out.
A Key Building Block for Modern Chemistry
Anyone who has set foot in a chemical lab knows that some compounds show up again and again in recipes for new molecules. 2-Bromopropionyl bromide is one of those reliable players. With two bromine atoms attached to a three-carbon chain, this chemical finds work as a building block in organic synthesis. Chemists grab it when they want to add a propionyl group to a molecule or introduce bromine atoms to shake up reactivity. The chemical’s direct nature and high reactivity transform it into a favorite for many synthetic steps. It's not glamorous work; just dependable and effective.
Pharmaceutical Roots: Where New Medicines Begin
Drug development runs on creativity and precision. Before a new medicine goes anywhere near a patient, it usually passes through the hands of several intermediate compounds. 2-Bromopropionyl bromide helps craft these intermediates. As a reagent, it enables the creation of chiral amino acids, special peptides, and active pharmaceutical ingredients (APIs) that fuel the next wave of treatments. These intermediates impact cancer drugs, antibiotics, and therapies that combat chronic illness. The stability and predictability of this chemical make long experiments more bearable—less time spent fighting reactivity headaches and more time focusing on discovery.
Making Polymers and New Materials
Down the street from pharma labs, polymer scientists use this compound to make fancy plastics and intelligent materials. Want a coating that responds to how much light it gets? Chemists turn to 2-bromopropionyl bromide to anchor reactive groups onto polymer chains. This approach customizes the physical properties—making new plastics stretchy, sticky, or resistant to bugs and sunlight. Some researchers go further, using this compound to build dendrimers, which look like tiny chemical trees that trap pollutants or carry drugs through the body. The science feels futuristic, but the process sits on a very practical workhorse.
A Tool for Life Science Breakthroughs
Biochemists spend days trying to shuffle atoms on proteins and small molecules. Protein labeling and surface modification often start with brominated reagents like 2-bromopropionyl bromide. By adding these tags, researchers can track how proteins fold, how drugs stick to enzymes, or how sensors detect disease. The compound’s reactive nature allows quick changes on a protein’s surface, unlocking experiments that help doctors and scientists spot illnesses earlier and understand biology at the atomic level.
Real-World Concerns and Responsible Chemistry
Anyone handling this brominated ingredient knows it comes with risks. The same reactivity that helps in the lab poses dangers if misused. Strict handling procedures cut down on spills and accidents. Many modern labs use fume hoods, double gloves, and airtight bottles, backed by training that comes from experience—one splash and you remember to respect the bottle. Environmental health groups also keep an eye on compounds like this, pushing for greener alternatives and safer waste disposal. Chemistry always involves trade-offs: innovation rides alongside responsibility.
Looking Ahead: Finding Greener Paths
Chemists now experiment with less hazardous replacements, aiming for sustainable processes. Academic labs and industry giants test new recipes, hoping to swap out old reagents without losing reliability. Treating safety and sustainability as part of good science isn’t just a trend—it’s what keeps the field moving forward. Innovations in reaction conditions, storage methods, and waste management continue to evolve, nudged by the lessons learned in every practical application.
Why Care About Storage and Handling?
Anyone who’s spent a little time in a laboratory knows stories don’t always start with grand experiments; they begin quietly, with small decisions like where a bottle sits and what glove you pick up. 2-Bromopropionyl bromide stands out as a clear example of why it pays to stay sharp. This compound usually shows up as a colorless liquid, but it packs a punch. Skipping steps in its storage or taking shortcuts during handling can turn routine tasks into an emergency.
The Hazards Hiding Behind the Label
Fumes from 2-bromopropionyl bromide sting, both the nose and lungs. Let’s not sugarcoat it—a single whiff has sent many running to an eyewash station or worse. Danger isn’t just about breathing though. Skin and eyes demand respect, and this chemical will burn. In my own university lab years, every new student knew exactly where the nearest fume hood stood before even opening a reagent bottle like this.
Storing 2-Bromopropionyl Bromide: Key Steps
I’ve found over the years that mistakes happen slowly at first: someone leaves a bottle too close to a window or forgets to tighten a lid. But these small errors add up. To avoid them, keep the bottle in a cool and dry place, far from direct sunlight or any source of heat. Moisture will trigger violent reactions, generating corrosive fumes. No one wants to open the lab door to clouds of hydrogen bromide. Only keep containers in a chemical storage cabinet that’s designed to resist acids and segregate from reactive compounds.
Shelves lined with absorbent pads help catch the odd drip. I always check that bottles have intact, tight-fitting lids—firm closures matter more than any warning sign on the door. No excuses for storing chemicals above eye level or next to strong bases, oxidizers, or water-reactive materials. Inventory checks every month keep storage honest and orderly.
Handling Practices That Make a Difference
Pulling on the right gloves becomes second nature with time—my personal pick for this compound has always been nitrile or neoprene, since latex offers little protection. Face shields and chemical splash goggles turn into more than a checklist item when you’ve seen a splash over someone’s glasses. In the lab, always carry out transfers in a properly ventilated fume hood. I make a point to run the fan for several minutes before uncapping corrosive bromides, just to be safe.
Never let new users work alone. Buddy systems save more headaches than people realize. Spill kits loaded with neutralizers should sit near work benches. Practice using them makes a big difference—no one reacts well to their first chemical spill if they’re unprepared. Double-checking procedures and reading through the safety data sheet helps too; this habit forms the backbone of any responsible research setting.
The Cost of Neglect
Stories of small spills turning into building evacuations remind everyone not to get complacent. Apart from health hazards, property damage and regulatory fines loom for those who get lazy. Labs that keep logs of every movement, practice double containment, and label everything in plain language avoid most disasters.
Building a Culture that Lasts
In all my years around chemicals, no protocol or fancy cabinet stands in the way of disaster as effectively as a culture of ownership. People who watch each other’s backs, take pride in clean workspaces, and aren’t afraid to point out mistakes keep everyone safer. Handling 2-bromopropionyl bromide may never turn routine, but with steady attention and respect, it certainly doesn’t have to end in tragedy.
The Real Hazards Behind the Label
Working with chemicals like 2-Bromopropionyl Bromide means stepping into a space where small mistakes can cause outsized problems. I’ve handled reactive acyl bromides in busy labs, and I’ll never forget the time a tiny splash ate a hole through an old pair of jeans—and left my leg with a nasty red spot before I could wash it off. This compound isn’t just an irritant; it attacks skin, eyes, and the respiratory system. Add water, and it hisses out hydrogen bromide, a gas that stings lungs and eyes. That pale yellow bottle is full of trouble for the unprepared.
Setting Up for Safety Starts Before Opening
Old chemical fume hoods, heavy airflow, and good lighting set the safe stage. Always check that sash glass moves smoothly and the airflow pulls in a Kleenex when tested. I’ve seen students ignore airflow alarms and end up coughing for hours. Have plenty of soap and water—nothing fancy, just within arm’s reach. Place an open bottle near an exit or in dead air and fumes linger around your face. Take it from experience, a strong hood is the most important “PPE” you’ll use.
The Right Gear Makes All the Difference
Every chemist knows about nitrile gloves—two pairs, not one. Goggles with side shields, not just over-the-glasses “fashion statements.” Lab coats that button closed, not tied at the waist. I once tried latex gloves for an acyl bromide—five minutes in and my thumb felt slippery and raw. Nitrile stayed intact. Add closed shoes, long sleeves, and a proper face shield if there’s any risk of splashing. Fitting this chemical into a process means extra steps, not shortcuts. Don’t trade comfort for convenience.
Storage and Handling
Some folks toss reactive bottles onto cluttered shelves, not thinking about what’s stacked below. This stuff needs a cool, dry spot—away from acids, amines, and bases. A leak near any of those, and you get smoke, heat, or worse. I once saw shelves warped where a bottle leaked unnoticed for a week. Use secondary containment. Good habits, like labeling with clear dates and hazards, cut through confusion when you’re tired or in a rush.
If Trouble Comes—Know How to Respond
Spills aren’t rare. Keep spill kits in sight—vermiculite, neutralizer, and an absorber just for acids. Alert everyone nearby, then deal with it head-on. If it lands on skin, wash for fifteen minutes under running water. Eyes get the same, but skip eyewash bottles—use a plumbed station. Breathing vapors means leaving the area and getting outdoor air. Don’t stick around to “see if it clears.” Call in trained help for large cleanups.
Proper Waste Disposal Can’t Wait
Every time 2-Bromopropionyl Bromide ends up in the waste bin without proper labeling, you invite disaster. Neutralize in the hood, separate halogenated waste, and treat every scrap of tubing, gloves, and filter paper as contaminated. I’ve seen folks get lazy, pour leftovers down the drain, and trigger fire alarms or worse. Comply with local regulations and lean on your institution’s environmental staff. That follow-through closes the loop safely.
Why Purity Matters for Buyers and End Users
Purity isn’t just a number or a promise on a label. It signals the level of care that went into making and handling a product. Talking with engineers and buyers over the years, most want clear proof about content. Food manufacturers, for example, might ask for at least 99% purity in a food-grade additive. A further decimal—like 99.9%—makes a world of difference on a lab bench making pharmaceuticals, or on a construction site needing consistency in a concrete additive.
Contamination or unwanted compounds spell trouble. They sneak past, and all sorts of complications follow. Imagine a lab trial ruined because an unexpected trace mineral changed the result—or a product recall that costs a company not only lost revenue, but trust. Purity reports backed by third-party labs, and strong documentation for each shipment, help avoid these disasters. Nobody enjoys uncertainty when someone’s health, a costly process, or millions of dollars ride on a decision.
Getting the Packaging Right
Packaging serves as the gatekeeper for quality. People rarely talk about it, but in my own work with both bulk chemicals and consumer goods, sturdy and thoughtful packaging protects the product before it ever reaches the customer. Nobody enjoys phone calls about torn sacks, spilled powder, or drums that leak during transport.
The right container keeps moisture, oxygen, and sunlight out, blocking the effects those elements bring—clumping, degradation, or contamination. When I’ve helped coordinate shipments sensitive to air, those bulk sacks were lined with polyethylene bags and sealed tight. A company sending laboratory-grade material usually relies on high-density polyethylene bottles or amber glass jars, sized to match order volume. Labels carry more than just the product name. They should show the batch number, production date, net weight, and safety warnings in clear print. This information lets anyone along the supply chain trace, handle, and store confidently.
Potential Solutions and Industry Practices
Shared language sets clear expectations for both sides. Standard benchmarks—such as ASTM for construction, FCC for food, or Pharma Grade for medicine—cut back on gray areas. I’ve watched contracts grind to a halt until specs matched, so spelling this out before any production starts saves grief later.
Some companies add QR codes or data loggers directly onto packaging. This step lets receivers check certificates, test results, or chain of custody right from the dock. I remember working with food companies who demanded digital access to each batch certificate before unloading any truck. The moment they spotted something unusual, the whole load got quarantined—no guesses, no shortcuts.
Another safeguard comes from working only with suppliers who document how they maintain both purity and packaging. This isn’t just about compliance. Plenty of major brands send their own inspectors to visit factories and warehouses, double-checking cleanliness and tamper-proof measures themselves.
Raising the Bar, Lowering the Risk
No one wants callbacks or ruined reputations from a shortcoming in purity or careless packaging. By insisting on detailed paperwork, trusted certifications, and tailored, sturdy containers, buyers gain confidence in every step. As digital tools and stricter standards keep spreading, mistakes and guesswork shrink—while safety, performance, and trust keep going up.