2-Bromobutyric Acid: Paths of Utility, Progress, and Caution
Historical Development
2-Bromobutyric acid grew out of the wave of synthetic chemistry that followed breakthroughs in carboxylic acid research in the late 19th century. Early organic chemists often leaned on trial and error, and the development of brominated acids opened doors in pharmaceuticals and agrochemicals. By the 1950s, researchers homed in on brominated acids as useful intermediates for building blocks in both lab and industrial settings. From the days when its potential was hinted at in handwritten lab notes, up until patent activity hinted at more practical applications, 2-bromobutyric acid’s track record is linked to laboratory persistence and the growing demand for specific, halogenated molecules.
Product Overview
Every bottle of 2-bromobutyric acid contains a substance commonly used by chemists aiming to alter molecules by adding new reactive sites. Its appeal stems from that active bromine group, which opens up plenty of chemical reactions whether someone's working on a small scale in a university or producing tonnes at an industrial plant. This acid plays an unglamorous, yet essential, role in the synthesis of complex molecules, especially ones used in medicine, biology, and materials research.
Physical & Chemical Properties
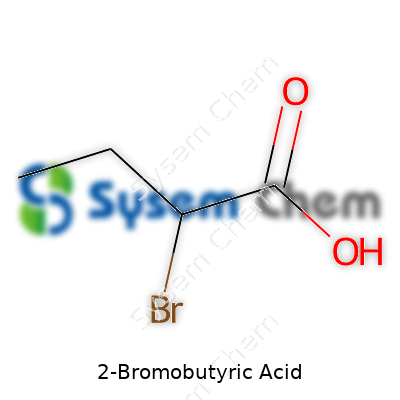
Seen on the bench, 2-bromobutyric acid appears as a colorless to faintly yellow liquid, with a sharp odor that never lets you forget you’re handling a halogenated organic. Its molecular formula is C4H7BrO2, and it boasts a molecular weight just north of 167 g/mol. The acidification makes it both water-soluble and easy to dissolve in many common organic solvents. Its boiling point, around the 200°C mark, and melting point near −15°C, put it squarely in the “liquid at room temp” camp, which simplifies handling in many labs. The molecule has a chiral center, so chiral synthesis and separation tasks pop up often for those looking to use it in specific pharmaceuticals or advanced materials.
Technical Specifications & Labeling
Labeling on a drum or flask of 2-bromobutyric acid should carry clear hazard warnings. Regulations call for details on purity (often 98% or more for lab uses), storage advice against sunlight and moisture, and a rundown of producers’ batch tracking. Researchers always watch for quotes of enantiomeric purity, since chiral acids can easily swap hands during synthesis. Chemical suppliers use identifiers like CAS number 600-28-8, making it easier to cross-check specs or communicate with colleagues across borders. A kit in a teaching lab often includes safety data, hazard pictograms, and brief reminders about handling.
Preparation Method
The mainstream preparation combines alkylation and halogenation steps, often starting with butyric acid or one of its derivatives. One common route reacts butyric acid with hydrobromic acid or phosphorus tribromide, swapping out a hydrogen for a bromine atom. Small-scale syntheses let chemists tweak reaction times, solvent, and temperature, but even at scale, the chemistry leans on straightforward reactivity between carboxylic acids and halogen sources. Some labs favor routes that cut down on side reactions or reduce the hazard of bromine vapors, using modern techniques like phase-transfer catalysis or milder reagents, which cater to greener practice.
Chemical Reactions & Modifications
The reason chemists reach for 2-bromobutyric acid again and again traces back to that bromine atom at the second carbon. Bromine in that spot welcomes nucleophilic substitution, sparking a variety of reactions that feed into pharmaceuticals, specialty polymers, or agrochemicals. Labs commonly turn this substrate into amino acids, carboxylate derivatives, or even more elaborate building blocks by swapping out the bromine. Stereochemically, 2-bromobutyric acid offers a playground for producing enantiomers, and catalytic asymmetric reactions thrive with it as a starting material. Grignard, reduction, and esterification reactions also fill notebooks and patents alike.
Synonyms & Product Names
On a shelf in any well-stocked chemical supply room, the bottle might sport names such as α-bromobutyric acid, 2-bromobutanoic acid, or their equivalents in other languages. Short chemical forms or catalog abbreviations like 2-BBA often crop up in supplier lists. Researchers check product data sheets for synonyms, since regulatory documents in one country may use a different moniker. Despite the name variations, chemists recognize the core utility it promises, and communication about such substances relies on a shared understanding of its shorthand.
Safety & Operational Standards
Few substances command as much respect in the lab as those containing both a carboxyl group and a reactive halogen. 2-Bromobutyric acid irritates skin, eyes, and lungs, with the bromine increasing potential for acute toxicity and environmental risk. Gloves, goggles, and fume hoods form the first line of defense. Labs display pictograms and handling reminders next to stock bottles. Chemical hygiene plans emphasize spill protocol, since traces can cause persistent problems in shared spaces. Industrial settings pay extra attention to ventilation, with local exhaust and air monitoring standing as routine. Emergency procedures train workers to respond rapidly to exposure or spills, aiming to reduce both human and environmental harm.
Application Area
2-Bromobutyric acid enjoys most of its use behind the scenes, feeding into processes that create drugs, agrochemicals, and biological probes. Its role as an intermediate in synthesizing chiral molecules sits near the top of its industrial value chain. Pharmaceutical researchers rely on it to produce molecules aimed at neurological disorders and metabolic pathways. The agricultural sector harnesses its chemistry to build herbicides or growth regulators. In academic settings, the acid’s predictable reactivity makes it a favorite for teaching students about nucleophilic substitution, chirality, and the practicalities of organohalide chemistry. Modern biotech companies lean on the acid as a platform for making molecules that need a chiral boost on short timelines.
Research & Development
Research on 2-bromobutyric acid seems forever in motion, as each year brings new synthetic strategies and enantioselective methods. Teams hunting for greener synthesis prefer solvent-free processes, biocatalysis, or milder halogenation agents. Chiral auxiliaries and catalytic systems tailor the production of one enantiomer, not the other, pushing the limits for pharmaceuticals where that choice makes or breaks drug safety. Analytical chemistry also invests in finding better ways to trace impurities, since the acid’s reactivity means batch-to-batch differences can arise. Universities fund projects to tweak the molecule for targeted biological effects, and startups build on R&D done decades before, proof that even a “basic” chemical can drive ongoing innovation.
Toxicity Research
Toxicological reports document both the knowns and the work still to be done for 2-bromobutyric acid. Animal studies show negative impacts on the nervous system, and cell culture work backs up its cytotoxic profile. As a halogenated organic, breakdown products enter water and soil, making environmental fate a topic for sustained research. Data suggest strict exposure limits for workers, and, in some countries, environmental agencies track emissions closely. Regulatory science deals with gaps by enforcing labeling, promoting closed-system handling, and encouraging research into biodegradability. The acid’s metabolic breakdown products also attract researchers interested in long-term human health risks or subtle effects on aquatic life. Actual human exposure remains rare outside chemical manufacturing, but guidelines echo a conservative, safety-first approach.
Future Prospects
The story of 2-bromobutyric acid will keep unfolding, shaped by both industry push and the evolving regulatory landscape. Demand looks set to grow wherever chiral intermediates tier up for pharmaceuticals or agrochemical innovation. Green chemistry and regulatory requirements should guide efforts to develop safer, more sustainable preparation methods. R&D likely pivots toward biodegradable analogs, smarter catalysts, and more selective processes. As machine learning and automation enhance organic synthesis, production may get faster and more sustainable. Decades of accumulated knowledge feed into every new bottle made, yet each year opens new questions on safety, environmental impact, or medicinal chemistry value—ensuring that 2-bromobutyric acid remains not just a lab staple but a subject of research.
In the Lab: A Reliable Intermediate
2-Bromobutyric acid finds steady use in organic synthesis. Many chemists reach for this compound to build carbon chains in pharmaceutical research. Its popularity comes from the way its bromine atom reacts with other molecules, sparking transformations that set up key steps in complex reactions. I’ve seen lab partners turn to 2-bromobutyric acid when they want to introduce butyric structures quietly and cleanly, especially in the early stages before branching off to trickier chemistry.
This compound offers a direct route to chiral centers—those building blocks that dictate the shape and behavior of lots of modern medicines. Once, while working through a synthesis challenge, I watched as colleagues used 2-bromobutyric acid to steer a reaction toward a precisely-shaped product needed for an experimental epilepsy drug. This kind of careful engineering can mean the difference between an active, effective therapy and one that fizzles in tests. Being able to start that process with reliable, predictable chemistry frees scientists to focus their attention where it counts.
Pharmaceutical Research and Chiral Compounds
The world of drug discovery leans heavily on intermediates like 2-bromobutyric acid. Its role shows up most in the formation of chiral molecules. The body often treats left- and right-handed versions of a molecule very differently—sometimes, only one form brings relief, while the other brings side effects. Teams designing new drugs use 2-bromobutyric acid again and again to aim for the right hand or the left, so to speak.
One leading example is the synthesis of anti-epileptic medicines, where precise arrangement of atoms can change how a drug controls neural activity. Making those chiral centers means thinking several steps ahead, and 2-bromobutyric acid becomes a tool for chemists striving to scale up small-batch successes to reliable, high-purity products.
Creating Building Blocks for Agrochemicals
Crops around the world depend on ever-better agrochemicals for protection and healthy growth. I’ve spoken to teams who rely on 2-bromobutyric acid to set up important side chains found in herbicides and fungicides. Its manageable size and functional groups mean chemists can splice it into different frameworks without wasting time on excessive purification steps. The result is more streamlined development of pesticides that target pests while sparing beneficial insects.
Looking at Safety and Environmental Concerns
Some risk comes with the power of a reactive intermediate. Handling 2-bromobutyric acid safely calls for clear protocols in the lab and the plant. A nose-full of this compound isn't pleasant, and it can irritate skin and eyes. In community discussions, I’ve noticed growing interest in recycling solvents and neutralizing leftover reagents when working with halogenated acids. Environmental watchdogs push for these steps because accidental releases can put pressure on waterways and soil.
Green chemistry offers several solutions—such as using closed systems, improved ventilation, and waste collection. There’s growing tech in the pipeline for breaking down brominated wastes before they reach the environment. It’s important to not only harvest the benefits of versatile acids like this one, but also to share responsibility for keeping people and places safe.
In Summary
2-Bromobutyric acid brings flexibility and reliability to research in medicine and agriculture. Its role as a chemical intermediate makes ambitious projects possible, from new drugs to safer crop treatments. Handling and disposal require vigilance, and the best scientific teams work as hard on safety as they do on reaction yields. With fresh tools and stricter protocols, labs and factories have a shot at balancing innovation with responsibility.
What Makes 2-Bromobutyric Acid Significant in Chemistry
Walking through any organic chemistry lab, you’ll spot all sorts of compounds on the shelves, and 2-Bromobutyric acid finds its place there for good reason. The chemical formula, C4H7BrO2, speaks to its molecular make-up: four carbons, seven hydrogens, a bromine atom, and two oxygens. The molecule weighs in at 167.00 g/mol. Not just another structure, this molecule captures both simplicity and functionality, offering plenty of value to experimental chemists.
Why Structure and Formula Matter in Real Labs
I’ve worked with plenty of four-carbon acids in research. One substitution or small tweak in a molecule—say, adding bromine—totally changes the outcome. 2-Bromobutyric acid stands out because the bromine atom opens doors for selective reactions. It often acts as a starter for building bigger, more complex molecules. Without knowing the true formula, a chemist could risk making costly mistakes in synthesis or analysis. Recipes in chemistry must be precise; one misplaced atom can spell disaster or, worse, danger.
Practical Applications Bring Relevance
In pharmaceutical chemistry, detail matters. The configuration of 2-Bromobutyric acid—where the bromine sits—turns it into a versatile intermediate. Labs use it to build drugs, test catalysts, and explore new biochemical pathways. Its presence in small-molecule design shows up in pain medicine, anticonvulsant research, and even specialty polymers. The molecular weight becomes crucial during calculations for reactions and scaling up in industry. Chemists turn grams and moles into actionable plans with accurate formulas and weights. A single slip could ruin an expensive batch, so attention to formula details keeps projects on track.
Risks and Safe Handling
Anyone who’s spent time in the lab knows a misstep in handling halogenated acids like 2-Bromobutyric acid can cause problems. The bromine atom brings higher reactivity and potential toxicity compared to its non-brominated cousins. Safety sheets always recommend gloves, eye protection, and proper ventilation. Companies and universities have seen accidents when someone overlooked these factors. Sticking to safety protocols is non-negotiable—protective gear saves skin, eyes, and long-term health.
Supporting Chemists and Researchers
Healthy skepticism toward reference material is part of my process. Even experienced chemists occasionally discover inconsistent listings for chemical weights in textbooks or online. Double-checking the formula (C4H7BrO2) and the molecular weight (167.00 g/mol) from reputable sources, like peer-reviewed journals or the PubChem database, is my rule. These checks keep experiment plans reliable and reproducible, which feeds back into better science and safer labs.
Bringing More Accountability and Knowledge to the Field
Details shape outcomes in chemistry as much as creativity does. Sharing and confirming data on even bread-and-butter compounds like 2-Bromobutyric acid pushes science forward. Giving new chemists accurate, readable facts—the real formula, the measured weight, clear handling advice—sets a high bar for reliable work. It makes science more accessible. With each experiment involving this acid, accuracy fuels discovery, and teamwork across the field safeguards both results and people.
Understanding What You Store
Anyone who walked through a lab filled with labeled bottles quickly learns respect for the risks that come with strong-smelling acids and unusual chemicals. 2-Bromobutyric acid, with its sharp scent, warns you by smell alone that this isn’t something to leave out in a warm room or near a busy hallway. Responsible storage cuts down on the chance of skin burns, fire, and even poisonous fumes that sneak out when a cap loosens. What matters here isn’t just the label—it's the daily safety routine.
Temperature and Environment Matter More Than You Think
Many chemists, including myself, make routine checks on room temperature and airflow. A cool, dry closet or flammables cabinet works well for this acid. Shelves away from sunlight and heat keep bottles from expanding or cracking. Moist air turns small spills into bigger problems, so a simple humidity monitor near storage areas makes the difference. Keeping a working air vent and a tight seal on each bottle keeps the room fresh and the chemical where it belongs.
Container Choices and Communication Save Lives
Glass stops 2-Bromobutyric acid from reacting with plastic, which sometimes melts or gets brittle over time. Tight screw caps are not just a suggestion—they keep the substance at bay, cutting down on dangerous fumes or spills. Over the years, I found that a good habit involves double-checking every bottle’s seal, especially after a rush job.
Labels do the heavy lifting here. Clear, print-big warnings tell everyone around what lurks inside. Safety symbols help the untrained or tired worker spot risk in a blink. Storing away from bases, strong oxidizers, and anything that can spark limits bad interactions. Keeping an updated chemical inventory in a shared log means fewer surprises when bottles move or run low, and fresher sets of eyes will always spot a broken seal before the next person ends up in the hospital.
Training and Emergency Readiness
Many accidents follow from rushed training or forgotten drills. I remember one spill in a neighboring lab because someone stored too much acid above eye level, missing the warning on not stacking heavy, volatile bottles too high. Regular training on safe handling and spill procedures, along with well-marked grab-and-go safety showers and eyewash stations, saves time and eyesight. Workers must know exactly where these are—every second counts after a spill.
Fire extinguishers and neutralizing agents (like sodium bicarbonate) should stand within arm's reach. A clear printed sheet with spill clean-up steps taped to the inside of storage cabinet doors acts as a practical backup to memory.
Practical Steps for a Safer Workplace
Safe storage of chemicals never happens by accident—it depends on method, vigilance, and honest communication among everyone in the lab. Prioritizing these customs protects not only researchers and students but also cleaners, supervisors, and the people who spend less time in the laboratory but still share the building’s air and space. Use the right containers, label well, stay cool, and double-check everything. Simple habits, learned from small mistakes, build up to a much safer space for all.
Why 2-Bromobutyric Acid Matters in a Lab
2-Bromobutyric acid usually crops up in organic synthesis, often playing a role in pharmaceutical research or specialty chemical production. Its structure—a butyric acid backbone with a bromine attached—makes it useful. At the same time, that bromine atom also raises the stakes for anyone working with it. I remember the sharp smell and the immediate sting in my nostrils during the first exposure in an old university lab. That encounter hammered home the importance of treating reagents like this with respect, no matter how routine the work.
Controlling Exposure: Real Hazards
2-Bromobutyric acid isn’t just a gentle irritant; its vapor and liquid forms can burn skin, eyes, and the respiratory tract. Lab reports and MSDS data document its corrosive nature. Skin contact often brings redness and pain, sometimes even blistering. Inhaled vapors can clearly irritate airways. Swallowing it does more damage—nausea, abdominal cramps, and depending on dose, much worse.
On top of the irritation risk, brominated organic compounds—including this one—sometimes cause longer-term issues with repeated exposure: dermatitis, sensitization, or even effects on organ function. Keeping these risks front-of-mind affects every step of how you approach work in the lab.
Precaution Starts with Planning
I always begin by reviewing the Safety Data Sheet. Labs that invest in real training—not just surface-level reviews—catch most accidents before they happen. Always use a certified fume hood when opening bottles or handling transfers. Even a quick weighing can lead to an eye-watering cloud, especially if the hood sash isn’t at the right height.
Gloves sound obvious, but not everyone grabs nitrile or butyl gloves, which actually stand up to brominated chemicals. I keep a couple spare boxes nearby because nobody wants to cut corners just because the latex box is closest. Chemical splash goggles fit tight—even if they feel bulky compared to normal glasses, they stop vapor and droplets from slipping behind regular lenses.
Contamination sneaks around on surfaces you don’t expect: weigh boats, spatulas, doorknobs. I learned to double up on cleaning protocols, using chlorine bleach for spills or glassware and not just plain soap and water. A double-check with pH paper on the cleaned surface isn’t overkill. Lab coats with closed sleeves protect street clothes and skin, especially cuffs, which always seem to find their way into unexpected splashes during transfers or pouring.
Emergency Planning: What Actually Works
The fastest showers and eyewash stations are worth nothing if you can’t get to them. I always do a “walk-through” at the start of each semester’s lab, timing that trip from workbench to eyewash or shower with eyes closed. Anyone new to the chemical shed should run through that drill.
Spills demand more than paper towels. I keep spill kits with neutralizer and absorbent for acids nearby. Ventilate the room, cordon off the exposure zone, and use proper waste bins lined with compatible plastic—not just the nearest trash.
Waste Disposal and Environmental Mindfulness
Disposal matters nearly as much as handling. Pouring leftover acid or contaminated debris down the drain creates a hazard both for workers and the surrounding environment. My lab contracts with waste services to collect brominated organics. If that’s not possible, double-containerize waste in sealed, labeled bottles, and log out each disposal on paper. The EPA and OSHA both set hard rules—compliance isn’t just about safety, but about keeping doors open to any lab or business working with hazardous materials.
Culture Over Compliance
Building a genuine culture of safety beats checking boxes. Sharing mistakes or near-misses openly helps everyone keep risk in perspective and makes room for new safety ideas. I’ve seen labs go years without a single incident, and it always comes down to that genuine attention—where every tech or scientist looks out for the next person. For chemicals like 2-Bromobutyric acid, there’s no shortcut to preparation, protective gear, and practiced response to emergencies. Awareness, backed by action, keeps everyone safe.
What Buyers Should Know About Purity Choices
People don’t usually wake up thinking about 2-Bromobutyric Acid, but anyone working in a lab or with chemical supplies figures it into their day. Purity matters for more than just getting a clean reaction—sometimes it’s the difference between success and wasted time. So, talking about grades isn’t a technical game; it’s about getting the right stuff for the job.
Lab Research Isn’t Like Paint-By-Numbers
In the lab, small impurities can throw off entire projects. Say you’re working toward a chiral intermediate or a pharmaceutical candidate, and you pull a bottle of low-purity 2-Bromobutyric Acid. Even a small amount of leftover starting material could spoil the run. I’ve watched colleagues go through months of synthesis only to realize an impurity from the first gram scaled up into a headache late in the process. In those moments, the paper trail leads right back to the purity label.
High-purity grades—think 98% or better—come at a steeper price, but the value shows when there’s less troubleshooting or guesswork. Technical grade or reagent grade sits just above crude preparations in terms of price. You still find traces of water, halogenated junk, or organic leftovers. For some folks in agriculture or those making bulk intermediates, these traces don’t spell disaster. But try taking that same technical grade into a sensitive pharma or biotech workflow. Instead of a smooth reaction, you get side-products and more questions during quality control.
Risk, Safety, and Compliance
Sometimes regulatory bodies set rules on acceptable traces in chemicals used for medicine, pesticides, or food. Most of this doesn’t make headlines, but governments have learned their lesson from past contamination scandals. For chemicals like 2-Bromobutyric Acid, documentation of grade isn’t just red tape. If the paper says “analytical grade,” but the bottle delivers surprises, safety gets compromised. Labs that run trace analysis want detailed certificates of analysis. That’s not just for show—sometimes it catches a batch gone wrong before anyone gets hurt, or it helps during an audit.
The Money Angle
Raw material costs eat into budgets in a hurry. In tight economic times, it’s easy to grab the cheapest grade and try to make do. But some users don’t realize extra purification work eats man-hours, solvents, and sometimes even more product. Pay less up front, pay more down the road. Factory managers and project leads learn—sometimes painfully—that “high enough” purity isn’t always enough.
How Buyers Can Protect Themselves
The quick fix? Talk directly with your supplier. Good vendors explain where their chemical comes from, how the purity number is measured, and what’s in the fine print. Asking hard questions before the shipment lands saves lab teams later scrambling for information. I always tell newcomers: Don’t lean only on a catalog description. Request a certificate of analysis for the actual batch. See if they’ll share details about testing—gas chromatography, NMR, or titration. It’s not about distrust; it’s smart diligence.
Chemical buyers in the know push for transparency. The right communication lines stop a lot of headaches. It’s not about buying the highest possible grade every time. It’s about understanding what the job demands and refusing to let purity become an afterthought.