2-Bromo-2-Methylpropionic Acid: A Close Look
Historical Development
After the mid-20th century brought rapid growth in polymer chemistry, many niche chemicals found their calling in large-scale industrial processes. 2-Bromo-2-methylpropionic acid emerged out of this same climate, looking almost tailor-made for specialties like atom transfer radical polymerization, where control over molecular weight and architecture matter. Early chemists, often working without modern safety equipment or regulatory oversight, combined traditional organic synthesis knowledge with a drive to build brand-new polymers. 2-Bromo-2-methylpropionic acid didn’t start in the limelight, but its halogenated backbone and carboxylic acid group soon attracted attention for modifying growing polymer chains with consistent, reliable results. Unlike some bench-top curiosities, its role in advancing controlled polymerization gave it a place that hasn't faded, anchoring further research in both academia and industry.
Product Overview
2-Bromo-2-methylpropionic acid stands out as a compact, versatile molecule. Its structure combines a bromo group and a carboxylic acid onto a branched three-carbon skeleton. Labs and factories turn to this chemical not only for its reactivity, but for what it enables in bigger projects. Plenty of suppliers keep it in stock with various levels of purity, because end users—ranging from research teams to plastics manufacturers—rely on a steady supply for custom syntheses or scale-up production. It's not the type of compound that dominates headlines, but for anyone designing specialty polymers or fine chemicals, 2-bromo-2-methylpropionic acid shows up just often enough to make it a familiar ingredient in the toolkit.
Physical & Chemical Properties
The acid presents as a white to off-white crystalline powder, with a melting point hovering around 49–51°C and a boiling point in the 215–217°C range under reduced pressure. Its solubility stretches from water to common organic solvents like methanol, ethanol, and ether, which simplifies purification and downstream reactions. The dense halogen in its structure makes it more reactive compared to other carboxylic acids, particularly in nucleophilic substitution. Storage stability rates as good, provided temperature and moisture stay under control, and its odor tells you—rather sharply—about its halogen content. From a handling perspective, gloves, goggles, and good ventilation do more than just tick a box: they keep accidental exposure at bay, protecting against corrosive and irritant effects.
Technical Specifications & Labeling
Manufacturers often bring 2-bromo-2-methylpropionic acid to market at a purity of 98% or higher, listing it with analytical data to back up the claim—think HPLC, GC-MS, and NMR spectra that help users confirm what’s in the bottle. Labels name the product, note the hazard statements, and carry warning symbols under the Globally Harmonized System, such as the exclamation mark for skin and eye irritation. Each bottle displays batch numbers for traceability, and spec sheets come with recommended storage conditions—usually between 2–8°C, in airtight containers shielded from light and air.
Preparation Method
The most common synthesis route involves bromination of pivalic acid, where temperature, solvent, and reaction speed shift the odds toward a clean product and little overbromination. Iodine or phosphorus tribromide sometimes enter the picture to convert precursor carboxylic acids into the bromo acid efficiently. Each step calls for a practical approach, from maintaining reaction temperatures with regular ice baths to quenching unreacted bromine with sodium thiosulfate. The crude acid then passes through washes with diluted mineral acid, brine, and drying agents such as magnesium sulfate before being recrystallized to the required purity.
Chemical Reactions & Modifications
2-Bromo-2-methylpropionic acid acts as a flexible starting point in organic synthesis. Its bromo group enables SN2 substitutions, letting chemists install amines, alkoxides, or even azides into the core structure. In polymer chemistry, the acid’s bromo atom opens the door for living free radical reactions, making it a favored initiator for constructing well-defined polymers. Chemical modifications like esterification, reduction to alcohols, or conversion to corresponding amides unlock new compounds. Base-promoted elimination leads to isobutyric acid derivatives, showing off its utility in making building blocks with subtle functional twists.
Synonyms & Product Names
Plenty of catalogues know it by synonyms: α-Bromo-α-methylpropionic acid and 2-Bromo-2-methylpropanoic acid most often pop up in chemical databases. Analysts refer to it by its CAS number 598-31-2 as a shorthand. Product monikers sometimes include “initiator-grade” to highlight its role in polymerization, or “reagent-grade” for general synthetic work. In the lab, a shorthand like BMPA or BMPrA sometimes shows up on glassware tape, keeping things efficient—especially when working with multiple acids and brominated chemicals.
Safety & Operational Standards
Handling this acid isn’t something to take lightly: the bromo group delivers a sting on skin contact, and inhaling dust or fumes results in coughing or respiratory distress. I’ve watched even experienced chemists treat the bottle with respect, donning gloves, goggles, and sometimes a lab coat snapped tight. Given that spills cause local corrosion, work usually happens in a fume hood with plenty of neutralizer close by. Laboratories and factories train staff on spill protocols, proper PPE use, and safe waste collection, keeping accidental exposure cases low. OSHA and REACH regulations have pushed for better labelling, documentation, and risk assessments, while safety data sheets include thorough first-aid and disposal instructions, leaving little room for oversight.
Application Area
The big draw comes in polymer chemistry, especially in atom transfer radical polymerization where the acid’s structure gives chemists control over how polymer chains start and grow. Specialty coatings, adhesives, and drug delivery materials trace their origins to well-controlled radical initiators based on 2-bromo-2-methylpropionic acid. Outside of plastics, it finds roles as a building block for pharmaceuticals, agrochemical intermediates, and even fine flavors or fragrances, each time providing a reactive group that tailors new molecules to an intended use. Production plants and research teams look at this acid when regular starting materials prove too inflexible for current goals, especially as the demand for high-value specialty materials keeps climbing.
Research & Development
Chemists keep pushing the boundaries on what 2-bromo-2-methylpropionic acid can do, developing greener synthesis routes using less hazardous chemicals or milder reaction conditions. There’s a constant search for better catalysts or solvent systems that cut waste and boost yields. In biomedical science, modified versions serve as handles for attaching drugs to polymers, allowing targeted delivery or slow release under physiological conditions. Patents describe new copolymers or surface treatments, showing a lively field that isn’t content to settle for the status quo. Even small improvements—like more stable, odorless derivatives—can ripple out, supporting industries from packaging to biomedicine.
Toxicity Research
The acid’s low-molecular-weight structure, combined with the bromo group, brings both acute and chronic toxicity concerns. Animal studies have documented skin and eye irritation, as well as possible effects on lungs after prolonged exposure. Some halogenated acids build up in tissues over time or generate hazardous byproducts during metabolic breakdown. Regulatory agencies continue to monitor for new data, while manufacturers include updated toxicity figures on safety data sheets as research emerges. Internally, research groups in academic and industrial settings run in-house screens for mutagenicity or ecotoxicity, aiming to head off problems before large-scale applications.
Future Prospects
Specialty chemicals like 2-bromo-2-methylpropionic acid look set to keep growing in value as new polymerization methods and custom functional materials take the stage. Greater push for green chemistry drives interest in recyclable initiators or bio-based feedstocks, with research teams seeking to replace hazardous steps without giving up performance. As electronic devices lean on specialty plastics for flexibility, heat resistance, or biodegradability, this small molecule supports bigger trends in sustainable design and high-precision manufacturing. Careers in chemical research and process scale-up will continue to rely on safe practices and solid technical knowledge, especially as application areas expand into medicine, sensors, and beyond.
Getting to the Basics
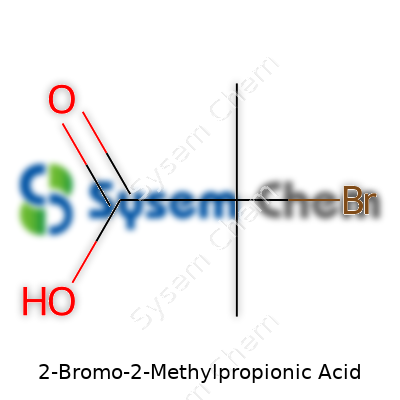
2-Bromo-2-methylpropionic acid carries the chemical formula C4H7BrO2. On paper, it looks simple enough: four carbon atoms, seven hydrogens, one bromine, and two oxygens. But beneath that tidy formula lies a story about how small tweaks in molecules can drive real-world applications and challenges — in labs, industries, and even in classrooms.
Breaking Down the Structure
The “bromo” in its name signals a bromine atom hanging on the second carbon in the propionic acid backbone. Add a methyl group in that same spot, and suddenly, you have a molecule that’s both highly reactive and distinct. Chemists recognize this sort of substitution instantly; it’s these little changes that let us fine-tune properties like polarity, solubility, and reactivity.
Why Bother with the Details?
Here’s why it pays to pay attention to structures like this: 2-Bromo-2-methylpropionic acid pops up in atom transfer radical polymerization (ATRP) — a controlled polymerization method. With ATRP, you get tighter control over molecular weights and shapes in plastics. The precise formula matters, because swapping even one atom can change a reaction’s outcome. In industries where uniform polymer chains mean better materials or medical devices, little tweaks make a huge difference.
From my own experience, academic labs fuss over the fine details of chemical formulas. Mix-ups happen — even to those with years under their belts — and they can set an entire experiment off track. Once, we thought we had the right isomer for a reaction, but a small substitution had been overlooked. What followed: a week of troubleshooting, late nights, and a lesson in paying close attention to formulas.
The E-E-A-T Factor in Chemistry
Google’s search guidelines push for Experience, Expertise, Authoritativeness, and Trustworthiness. Chemistry, as a field, rewards the same values — you don’t improvise on formulas and expect success. Years in the lab taught me to double-check chemical structures, learning from mistakes. Accurate knowledge helps professionals match safety procedures and choose reagents, reducing expensive or hazardous errors. Trust grows through verified information and repeatable results.
In the Classroom & the Lab
A clear understanding of formulas like C4H7BrO2 trains future scientists. Students memorizing it aren’t just cramming for exams—they’re building a toolkit for molecular problem-solving. Knowing where the bromine and methyl groups sit helps them predict how the acid reacts, interacts, and bonds. The more confident a person is with these details, the less likely they’ll misread a protocol or mishandle a dangerous substance.
Tackling Mistakes and Moving Forward
Mistakes surround chemistry — not out of carelessness, but because molecules are complex. Better resources, clear diagrams, and helpful digital tools can close the gap between theory and practice. Digital databases help avoid errors and reinforce accuracy. I’ve seen students light up when a structural model finally clicks, and they realize why X reacts with Y or why safety gear is non-negotiable.
What Really Matters
The chemical formula for 2-bromo-2-methylpropionic acid is more than numbers and letters. It represents a structure engineered for modern science and industry, with each atom in its place for a reason. Paying attention to such detail isn’t just good science — it’s how discoveries happen and how future chemists build confidence, one molecular model at a time.
Why Chemists Rely on 2-Bromo-2-Methylpropionic Acid
If you spend time in a lab or around folks who develop specialty polymers or advanced materials, you’ve probably seen 2-Bromo-2-Methylpropionic Acid pop up. Its structure offers a unique mix of bromo and carboxylic groups, which makes it a useful tool for driving reactions. Most people won’t see this compound outside of research and production. Behind the scenes, though, it quietly powers the creation of things you use every day.
One of the most important uses comes from its role as an initiator and building block in the world of controlled radical polymerization. Imagine you’re making plastics that need specific properties—maybe you want a surface to be super slick for medical tubing, or to have the right “grip” for an electronics application. 2-Bromo-2-Methylpropionic Acid helps create polymers with end groups that can do these jobs. In atom transfer radical polymerization (ATRP), scientists take advantage of its reliable reactivity to control molecular weight and fine-tune material performance.
Let’s Talk Personal Experience
During my time working with coatings and adhesives, I ran into the need for precision time and again. Some customers asked for coatings that repel water so thoroughly, it seemed like nothing could stick. Others wanted surfaces that bonded easily. Products built from well-constructed polymers, started with molecules like 2-Bromo-2-Methylpropionic Acid, delivered what they needed. I’ve seen manufacturers use it to anchor “smart” properties onto plastics—like adding sites where other chemical groups can latch on. This approach opens the door for drug delivery systems, diagnostic devices, and materials that sense their environment.
It’s not just about high-tech uses. While polymers often grab the headlines, 2-Bromo-2-Methylpropionic Acid steps up in making additives for lubricants, surfactants, and flame-retardants. With global regulations pointing to safer compounds, chemists turn to new synthetic routes, and starting materials like this compound often make their shortlists. Over the years, I’ve seen a push in the industry for raw materials that balance safety and performance, and every component counts.
Addressing Safety and Environmental Questions
Nobody working with chemicals ignores questions about safety and waste. Handling 2-Bromo-2-Methylpropionic Acid calls for careful storage, good ventilation, and personal protection. Bromo compounds can present risks—reactions can sometimes release fumes or leave residues you don’t want in your supply chain. Regulatory bodies keep a tight watch on how materials get blended, shipped, and disposed of. For teams planning to scale up use, treating effluents and reducing byproducts should be part of the budget from day one.
I often tell young researchers that planning greener syntheses pays dividends. By using more efficient catalysts, recycling solvents where possible, and treating brominated waste properly, both small and large labs address environmental expectations and keep communities healthier. Companies working in the US, Europe, or Asia all face similar pressure to document safety testing and follow rules for transport and worker protection.
Looking Forward
There’s a direct connection between a chemist’s choices and the things that end up in a consumer’s hands. As demand for precision and safety in plastics, electronics, and coatings continues to rise, compounds like 2-Bromo-2-Methylpropionic Acid will show up in more product development pipelines. Clear research, a focus on sustainability, and strong worker education keep this process on track.
Why Proper Care Matters
In the lab, it’s easy to overlook some of the less dramatic chemicals in favor of those that foam, smoke, or spark. But even a colorless, crystalline compound like 2-Bromo-2-methylpropionic acid can turn into a real headache if it isn’t stored or handled thoughtfully. Those who have worked with organic acids recognize the steady wear small missteps can take on a workspace—and on the people working there.
Storage: Keeping It Secure and Stable
2-Bromo-2-methylpropionic acid starts to degrade when exposed to light, moisture, or elevated heat. People often store it in tightly sealed glass containers. A desiccator pulls double duty, protecting both the material and anyone who handles it. It’s best to designate a cool, shaded shelf, far from sources of heat or sunlight, because this compound does not appreciate swings in temperature. I’ve watched seasoned chemists keep these acids on the lowest shelf just in case a jar ever breaks—nothing falls uphill.
Acids like this one shouldn’t mix with bases, acids, or oxidizers outside a controlled reaction. A shelf away from strong oxidizers or metal powders cuts down on the risk of dangerous reactions. Clear labels and dated containers can prevent awkward conversations in the lab and help avoid surprises years later.
Safety Around the Bench
2-Bromo-2-methylpropionic acid irritates the skin and eyes, so nobody in a functioning lab skips gloves. Nitrile gloves hold up against it, but I always double-check for pinholes—nobody enjoys that tingle when acid hits a paper cut. Eye protection feels obvious, yet every year I see someone squint and splash a drop, scrambling for an eyewash station.
Fume hoods are more than window dressing for this sort of material. The compound releases fumes, and nobody wants to breathe that in day after day. Even brief exposure can build up over time, leaving you with headaches or worse. Local exhaust, scrubbers, and closed vials keep the air cleaner for everyone in the workspace. For years, I’ve watched teams get careless only to deal with lingering coughs later in the week.
Dealing With Spills and Disposal
No matter how careful someone acts, spills will happen. Absorbent pads or sand catch liquid quickly, while acid-resistant gloves and goggles stay essential. Residue gets gathered in a labeled hazardous waste container. 2-Bromo-2-methylpropionic acid deserves special disposal procedures since it can’t go down the drain or into regular trash. A certified waste handler picks it up, protecting both people and waterways.
A leak or jar break might seem routine. In my time assisting in university teaching labs, we always reviewed the spill plan in safety meetings—neutralizer on hand, waste bin ready, no improvising. With dangerous materials, a predictable response saves a lot of trouble later.
Building Better Habits
What stands out: safe storage and careful handling become habits over time. Anyone with stories from a lab knows the value in a routine—checking glove integrity, using a fume hood for transfers, double-sealing jars. Sharing those habits with new faces keeps labs safer in the long run. People working with 2-Bromo-2-methylpropionic acid build a culture where small routines prevent big problems, keeping both their health and their research on track.
Chemicals in the Lab Aren’t Just Numbers and Formulas
2-Bromo-2-methylpropionic acid rarely turns up outside specialty labs and industrial environments. It grabs the attention of chemists for its role in controlled radical polymerization, which brings us the advanced plastics and coatings found in all sorts of products. The untrained eye sees tightly sealed bottles with warnings—those aren’t just for show. Through years in research labs, I’ve learned each label tells a story about real risk, not just red tape.
Direct Hazards Tied to 2-Bromo-2-Methylpropionic Acid
This chemical carries enough bite to make anyone cautious. Direct contact may irritate skin, eyes, and the respiratory tract. Inhalation of powder or fumes can trigger coughing or a sore throat. Skin exposure might lead to redness or even burns. Accidentally getting a splash in your eyes? That calls for immediate rinsing and a visit to a doctor. Outside of anecdote, the European Chemicals Agency lists it with warnings about both skin and eye irritation. Scientific suppliers state clearly that working without gloves and goggles isn’t just reckless—it’s asking for trouble.
Toxicity and Long-term Health Concerns
No one tests these niche chemicals on humans, so we lean on animal studies and chemical similarities for guidance. Chemists point out the bromo group in its structure, meaning it potentially packs more of a toxic punch than a simple carboxylic acid. Swallowed, it creates a risk of stomach pain and vomiting. In larger quantities, brominated chemicals have sometimes shown effects on liver and nervous systems. The risk may remain low for those working with tiny quantities in well-ventilated hoods, but risk doesn’t just disappear with careful technique.
Environmental Impact
Down the drain, chemicals like 2-bromo-2-methylpropionic acid travel through pipes and end up in places where they shouldn’t—streams, soil, or the local waste facility. Unlike the soap or juice we pour away after a meal, persistent halogenated acids may linger in the environment. They don’t break down easily. Aquatic organisms can be sensitive to this class of compounds. That matters in towns where research labs or factories sit near waterways. Waste disposal guidelines from environmental agencies become especially strict for this very reason.
Solutions Are Straightforward—Respect the Science
I’ll never forget the day a careless technician triggered an evacuation because a bottle was left unsealed and fumes drifted. This kind of mistake invites real danger. Responsible handling starts with education. Training people to know why a chemical matters for safety, not just how to open the bottle, makes the biggest difference. Every lab must enforce the use of proper gloves, goggles, and fume hoods—not as a formality, but as the baseline for all work. Build in regular inspections to catch unsafe habits early. Anyone working with this acid needs a ready supply of spill kits and a clear spill response plan. And don’t let the environmental piece slide—waste containers must be dedicated, labeled, and collected by certified professionals.
Every Chemical Deserves Respect
2-Bromo-2-methylpropionic acid calls for the same level of care as any lab-scale hazard. It may not be the most famous lab toxin, but it carries real risks. Science jobs reward those who don’t cut corners. Better safety today means fewer accidents and clean water for tomorrow. That lesson outlasts a single experiment every time.
Understanding Chemical Quality
Buying 2-Bromo-2-Methylpropionic Acid for lab or manufacturing use always puts purity front and center. Labs need confidence that every bottle on the shelf delivers what’s promised on the label, especially for specialty chemicals used in research or precise industrial synthesis. You’ll find this compound in the toolkit of anyone working on controlled polymer synthesis, RAFT polymerization, or even pharmaceutical intermediates.
Talking with colleagues, one finds most suppliers target a typical purity of 97% or better for this compound. Some go higher, hitting 98% to 99% for high-end applications. This isn’t just marketing: even small impurities can derail delicate reactions, trigger regulatory headaches, or introduce noise in analytical methods. Knowing this from troubleshooting countless failed syntheses, I never overlook the fine print on purity certificates.
How Purity Gets Reported
Reputable vendors publish purity on their technical data sheets, supported by analytical paperwork, such as HPLC or NMR printouts. Many will feature a “purity by GC” line, since gas chromatography can spot most process-related impurities.
Sometimes, a listing might read “min. 97% GC” or something similar. That minimum marks the threshold for what goes in the bottle, though batches may exceed it. The remaining 3% usually consists of trace solvents, water, or closely related by-products.
If you see 95%, that’s a red flag for any sensitive work. Some shortcuts crop up in the supply chain, where suppliers bulk up lower-purity lots. Genuine chemical distributors always provide a certificate of analysis (CoA) for every batch, which points out detected impurities by class or name.
Why Purity Matters Beyond the Lab
Even a small amount of brominated by-product can sometimes cause downstream headaches. I’ve seen plenty of projects delayed because off-spec reagents crept into larger scale production, making it hard to pass QC benchmarks or meet country-specific safety standards. Some polymer chemists find a lot of variance in molecular weight control if their initiator purity wobbles—even by a single percent.
If the goal is drug development, purity standards go even higher. Pharmaceutical grade lots often push to 99% and above, with individual impurity levels capped below 0.5%. Regulatory agencies worldwide expect this information on every shipment.
What to Ask Before Buying
It’s not enough to pick the highest number off the shelf. Ask your supplier for the full analytical workup: GC traces, NMR, even water content by Karl Fischer if moisture is a concern for the synthesis. Some labs I’ve worked in insist on seeing impurities broken down—especially if any potential residual solvents could trip up downstream processing.
Don’t rely on catalog promises alone. Request recent batch analysis, not just the generic specifications from a two-year-old document. Have a contingency plan for re-purification if the batch doesn’t meet your in-house requirements. Scrutiny here pays off, since sorting out impurities after the fact can eat up budgets fast.
Improving Confidence and Reducing Risk
For sensitive projects, partner with established suppliers who offer transparent batch history and allow returns on out-of-spec product. Avoid vendors with sketchy documentation or inconsistent QC processes. Some companies offer custom purification if the standard purity doesn’t cut it—sometimes well worth the lead time.
In summary, the standard purity for 2-Bromo-2-Methylpropionic Acid out there hovers around 97% and up, with some producers hitting even tighter specs. Stay skeptical, check every certificate, and communicate directly with vendors to lock in the right quality for your work.